Brand Name: Glucovance
Generic Name: (glyburide and metformin HCl)
Contents:
Description
Clinical Pharmacology
Indications and Usage
Contraindications
Warnings
Precautions
Adverse Reactions
Overdosage
Dosage and Administration
How Supplied
Glucovance patient information (in plain English)
Description
Glucovance® (Glyburide and Metformin HCl) Tablets contains 2 oral antihyperglycemic drugs used in the management of type 2 diabetes, glyburide and metformin hydrochloride.
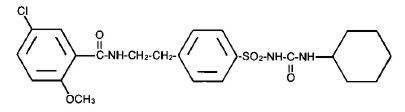
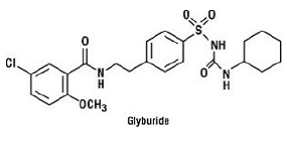
Glyburide is an oral antihyperglycemic drug of the sulfonylurea class. The chemical name for glyburide is 1-[[p-[2-(5-chloro-o-anisamido)ethyl]phenyl]sulfonyl]-3-cyclo-hexylurea. Glyburide is a white to off-white crystalline compound with a molecular formula of C23H28ClN3O5S and a molecular weight of 494.01. The glyburide used in Glucovance has a particle size distribution of 25% undersize value not more than 6 µm, 50% undersize value not more than 7 to 10 µm, and 75% undersize value not more than 21 µm. The structural formula is represented below.
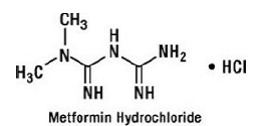
Metformin hydrochloride is an oral antihyperglycemic drug used in the management of type 2 diabetes. Metformin hydrochloride (N,N-dimethylimidodicarbonimidic diamide monohydrochloride) is not chemically or pharmacologically related to sulfonylureas, thiazolidinediones, or α-glucosidase inhibitors. It is a white to off-white crystalline compound with a molecular formula of C4H12ClN5 (monohydrochloride) and a molecular weight of 165.63. Metformin hydrochloride is freely soluble in water and is practically insoluble in acetone, ether, and chloroform. The pKa of metformin is 12.4. The pH of a 1% aqueous solution of metformin hydrochloride is 6.68. The structural formula is as shown:
Glucovance is available for oral administration in tablets containing 1.25 mg glyburide with 250 mg metformin hydrochloride, 2.5 mg glyburide with 500 mg metformin hydrochloride, and 5 mg glyburide with 500 mg metformin hydrochloride. In addition, each tablet contains the following inactive ingredients: microcrystalline cellulose, povidone, croscarmellose sodium, and magnesium stearate. The tablets are film coated, which provides color differentiation.
top
Clinical Pharmacology
Mechanism of Action
Glucovance combines glyburide and metformin hydrochloride, 2 antihyperglycemic agents with complementary mechanisms of action, to improve glycemic control in patients with type 2 diabetes.
Glyburide appears to lower blood glucose acutely by stimulating the release of insulin from the pancreas, an effect dependent upon functioning beta cells in the pancreatic islets. The mechanism by which glyburide lowers blood glucose during long-term administration has not been clearly established. With chronic administration in patients with type 2 diabetes, the blood glucose lowering effect persists despite a gradual decline in the insulin secretory response to the drug. Extrapancreatic effects may be involved in the mechanism of action of oral sulfonylurea hypoglycemic drugs.
Metformin hydrochloride is an antihyperglycemic agent that improves glucose tolerance in patients with type 2 diabetes, lowering both basal and postprandial plasma glucose. Metformin hydrochloride decreases hepatic glucose production, decreases intestinal absorption of glucose, and improves insulin sensitivity by increasing peripheral glucose uptake and utilization.
Pharmacokinetics
Absorption and Bioavailability
Glucovance
In bioavailability studies of Glucovance 2.5 mg/500 mg and 5 mg/500 mg, the mean area under the plasma concentration versus time curve (AUC) for the glyburide component was 18% and 7%, respectively, greater than that of the Micronase® brand of glyburide coadministered with metformin. The glyburide component of Glucovance, therefore, is not bioequivalent to Micronase®. The metformin component of Glucovance is bioequivalent to metformin coadministered with glyburide.
Following administration of a single Glucovance 5 mg/500 mg tablet with either a 20% glucose solution or a 20% glucose solution with food, there was no effect of food on the Cmax and a relatively small effect of food on the AUC of the glyburide component. The Tmax for the glyburide component was shortened from 7.5 hours to 2.75 hours with food compared to the same tablet strength administered fasting with a 20% glucose solution. The clinical significance of an earlier Tmax for glyburide after food is not known. The effect of food on the pharmacokinetics of the metformin component was indeterminate.
Glyburide
Single-dose studies with Micronase® tablets in normal subjects demonstrate significant absorption of glyburide within 1 hour, peak drug levels at about 4 hours, and low but detectable levels at 24 hours. Mean serum levels of glyburide, as reflected by areas under the serum concentration-time curve, increase in proportion to corresponding increases in dose. Bioequivalence has not been established between Glucovance and single ingredient glyburide products.
Metformin Hydrochloride
The absolute bioavailability of a 500 mg metformin hydrochloride tablet given under fasting conditions is approximately 50% to 60%. Studies using single oral doses of metformin tablets of 500 mg and 1500 mg, and 850 mg to 2550 mg, indicate that there is a lack of dose proportionality with increasing doses, which is due to decreased absorption rather than an alteration in elimination. Food decreases the extent of and slightly delays the absorption of metformin, as shown by approximately a 40% lower peak concentration and a 25% lower AUC in plasma and a 35-minute prolongation of time to peak plasma concentration following administration of a single 850 mg tablet of metformin with food, compared to the same tablet strength administered fasting. The clinical relevance of these decreases is unknown.
Distribution
Glyburide
Sulfonylurea drugs are extensively bound to serum proteins. Displacement from protein binding sites by other drugs may lead to enhanced hypoglycemic action. In vitro, the protein binding exhibited by glyburide is predominantly non-ionic, whereas that of other sulfonylureas (chlorpropamide, tolbutamide, tolazamide) is predominantly ionic. Acidic drugs such as phenylbutazone, warfarin, and salicylates displace the ionic-binding sulfonylureas from serum proteins to a far greater extent than the non-ionic binding glyburide. It has not been shown that this difference in protein binding results in fewer drug-drug interactions with glyburide tablets in clinical use.
Metformin Hydrochloride
The apparent volume of distribution (V/F) of metformin following single oral doses of 850 mg averaged 654 ±358 L. Metformin is negligibly bound to plasma proteins. Metformin partitions into erythrocytes, most likely as a function of time. At usual clinical doses and dosing schedules of metformin, steady state plasma concentrations of metformin are reached within 24 to 48 hours and are generally
Metabolism and Elimination
Glyburide
The decrease of glyburide in the serum of normal healthy individuals is biphasic; the terminal half-life is about 10 hours. The major metabolite of glyburide is the 4-trans-hydroxy derivative. A second metabolite, the 3-cis-hydroxy derivative, also occurs. These metabolites probably contribute no significant hypoglycemic action in humans since they are only weakly active (1/400 and 1/40 as active, respectively, as glyburide) in rabbits. Glyburide is excreted as metabolites in the bile and urine, approximately 50% by each route. This dual excretory pathway is qualitatively different from that of other sulfonylureas, which are excreted primarily in the urine.
Metformin Hydrochloride
Intravenous single-dose studies in normal subjects demonstrate that metformin is excreted unchanged in the urine and does not undergo hepatic metabolism (no metabolites have been identified in humans) nor biliary excretion. Renal clearance (see Table 1) is approximately 3.5 times greater than creatinine clearance, which indicates that tubular secretion is the major route of metformin elimination. Following oral administration, approximately 90% of the absorbed drug is eliminated via the renal route within the first 24 hours, with a plasma elimination half-life of approximately 6.2 hours. In blood, the elimination half-life is approximately 17.6 hours, suggesting that the erythrocyte mass may be a compartment of distribution.
Special Populations
Patients With Type 2 Diabetes
Multiple-dose studies with glyburide in patients with type 2 diabetes demonstrate drug level concentration-time curves similar to single-dose studies, indicating no buildup of drug in tissue depots.
In the presence of normal renal function, there are no differences between single- or multiple-dose pharmacokinetics of metformin between patients with type 2 diabetes and normal subjects (see Table 1), nor is there any accumulation of metformin in either group at usual clinical doses.
Hepatic Insufficiency
No pharmacokinetic studies have been conducted in patients with hepatic insufficiency for either glyburide or metformin.
Renal Insufficiency
No information is available on the pharmacokinetics of glyburide in patients with renal insufficiency.
In patients with decreased renal function (based on creatinine clearance), theplasma and blood half-life of metformin is prolonged and the renal clearance is decreased in proportion to the decrease in creatinine clearance (see Table 1; also, see WARNINGS).
Geriatrics
There is no information on the pharmacokinetics of glyburide in elderly patients.
Limited data from controlled pharmacokinetic studies of metformin in healthy elderly subjects suggest that total plasma clearance is decreased, the half-life is prolonged, and Cmax is increased, compared to healthy young subjects. From these data, it appears that the change in metformin pharmacokinetics with aging is primarily accounted for by a change in renal function (see Table 1). Metformin treatment should not be initiated in patients ≥80 years of age unless measurement of creatinine clearance demonstrates that renal function is not reduced.
Table 1: Select Mean ( ±S.D.) Metformin Pharmacokinetic Parameters Following Single or Multiple Oral Doses of Metformin
Subject Groups: Metformin Dosea
(number of subjects) | Cmaxb
( µg/mL) | Tmaxc
(hrs) | Renal Clearance
(mL/min) |
|---|
| a All doses given fasting except the first 18 doses of the multiple-dose studies |
| b Peak plasma concentration |
| c Time to peak plasma concentration |
| d SD=single dose |
| e Combined results (average means) of 5 studies: mean age 32 years (range 23-59 years) |
| f Kinetic study done following dose 19, given fasting |
| g Elderly subjects, mean age 71 years (range 65-81 years) |
| h CLcr=creatinine clearance normalized to body surface area of 1.73 m2 |
| Healthy, nondiabetic adults: | | | |
| 500 mg SDd (24) | 1.03 ( ±0.33) | 2.75 ( ±0.81) | 600 ( ±132) |
| 850 mg SD (74)e | 1.60 ( ±0.38) | 2.64 ( ±0.82) | 552 ( ±139) |
| 850 mg t.i.d. for 19 dosesf (9) | 2.01 ( ±0.42) | 1.79 ( ±0.94) | 642 ( ±173) |
| Adults with type 2 diabetes: | | | |
| 850 mg SD (23) | 1.48 ( ±0.5) | 3.32 ( ±1.08) | 491 ( ±138) |
| 850 mg t.i.d. for 19 dosesf (9) | 1.90 ( ±0.62) | 2.01 ( ±1.22) | 550 ( ±160) |
| Elderlyg, healthy nondiabetic adults: | | | |
| 850 mg SD (12) | 2.45 ( ±0.70) | 2.71 ( ±1.05) | 412 ( ±98) |
| Renal-impaired adults: 850 mg SD | | | |
| Mild (CLcrh 61-90 mL/min) (5) | 1.86 ( ±0.52) | 3.20 ( ±0.45) | 384 ( ±122) |
| Moderate (CLcr 31-60 mL/min) (4) | 4.12 ( ±1.83) | 3.75 ( ±0.50) | 108 ( ±57) |
| Severe (CLcr 10-30 mL/min) (6) | 3.93 ( ±0.92) | 4.01 ( ±1.10) | 130 ( ±90) |
Pediatrics
After administration of a single oral GLUCOPHAGE® (metformin hydrochloride) 500 mg tablet with food, geometric mean metformin Cmax and AUC differed less than 5% between pediatric type 2 diabetic patients (12 to 16 years of age) and gender- and weight-matched healthy adults (20 to 45 years of age), all with normal renal function.
After administration of a single oral Glucovance tablet with food, dose-normalized geometric mean glyburide Cmax and AUC in pediatric patients with type 2 diabetes (11 to 16 years of age, n=28, mean body weight of 97 kg) differed less than 6% from historical values in healthy adults.
Gender
There is no information on the effect of gender on the pharmacokinetics of glyburide.
Metformin pharmacokinetic parameters did not differ significantly in subjects with or without type 2 diabetes when analyzed according to gender (males=19, females=16). Similarly, in controlled clinical studies in patients with type 2 diabetes, the antihyperglycemic effect of metformin was comparable in males and females.
Race
No information is available on race differences in the pharmacokinetics of glyburide.
No studies of metformin pharmacokinetic parameters according to race have been performed. In controlled clinical studies of metformin in patients with type 2 diabetes, the antihyperglycemic effect was comparable in whites (n=249), blacks (n=51), and Hispanics (n=24).
Clinical Studies
Patients with Inadequate Glycemic Control on Diet and Exercise Alone
In a 20-week, double-blind, multicenter U.S. clinical trial, a total of 806 drug-naive patients with type 2 diabetes, whose hyperglycemia was not adequately controlled with diet and exercise alone (baseline fasting plasma glucose [FPG]
Table 2: Placebo- and Active-Controlled Trial of Glucovance in Patients with Inadequate Glycemic Control on Diet and Exercise Alone: Summary of Trial Data at 20 Weeks
| | Placebo | Glyburide
2.5 mg
tablets | Metformin
500 mg
tablets | Glucovance
1.25 mg/250 mg
tablets | Glucovance
2.5 mg/500 mg
tablets |
|---|
| a p<0.001 |
| b p<0.05 |
| c p=NS |
| Mean Final Dose | 0 mg | 5.3 mg | 1317 mg | 2.78 mg/557 mg | 4.1 mg/824 mg |
| Hemoglobin A1c | N=147 | N=142 | N=141 | N=149 | N=152 |
| Baseline Mean (%) | 8.14 | 8.14 | 8.23 | 8.22 | 8.20 |
| Mean Change from Baseline | −0.21 | −1.24 | −1.03 | −1.48 | −1.53 |
| Difference from Placebo | | −1.02 | −0.82 | −1.26a | −1.31a |
| Difference from Glyburide | | | | −0.24b | −0.29b |
| Difference from Metformin | | | | −0.44b | −0.49b |
| Fasting Plasma Glucose | N=159 | N=158 | N=156 | N=153 | N=154 |
| Baseline Mean FPG (mg/dL) | 177.2 | 178.9 | 175.1 | 178 | 176.6 |
| Mean Change from Baseline | 4.6 | −35.7 | −21.2 | −41.5 | −40.1 |
| Difference from Placebo | | −40.3 | −25.8 | −46.1a | −44.7a |
| Difference from Glyburide | | | | −5.8c | −4.5c |
| Difference from Metformin | | | | −20.3c | −18.9c |
Body Weight Mean
Change from Baseline | −0.7 kg | +1.7 kg | −0.6 kg | +1.4 kg | +1.9 kg |
Final HbA1c
Distribution (%) | N=147 | N=142 | N=141 | N=149 | N=152 |
| <7% | 19.7% | 59.9% | 50.4% | 66.4% | 71.7% |
| ≥7% and <8% | 37.4% | 26.1% | 29.8% | 25.5% | 19.1% |
| ≥8% | 42.9% | 14.1% | 19.9% | 8.1% | 9.2% |
Treatment with Glucovance resulted in significantly greater reduction in HbA1c and postprandial plasma glucose (PPG) compared to glyburide, metformin, or placebo. Also, Glucovance therapy resulted in greater reduction in FPG compared to glyburide, metformin, or placebo, but the differences from glyburide and metformin did not reach statistical significance.
Changes in the lipid profile associated with Glucovance treatment were similar to those seen with glyburide, metformin, and placebo.
The double-blind, placebo-controlled trial described above restricted enrollment to patients with HbA1c <11% or FPG
Patients with Inadequate Glycemic Control on Sulfonylurea Alone
In a 16-week, double-blind, active-controlled U.S. clinical trial, a total of 639 patients with type 2 diabetes not adequately controlled (mean baseline HbA1c 9.5%, mean baseline FPG 213 mg/dL) while being treated with at least one-half the maximum dose of a sulfonylurea (e.g., glyburide 10 mg, glipizide 20 mg) were randomized to receive glyburide (fixed dose, 20 mg), metformin (500 mg), Glucovance 2.5 mg/500 mg, or Glucovance 5 mg/500 mg. The doses of metformin and Glucovance were titrated to a maximum of 4 tablets daily as needed to achieve FPG
Table 3: Glucovance in Patients with Inadequate Glycemic Control on Sulfonylurea Alone: Summary of Trial Data at 16 Weeks
| | Glyburide
5 mg
tablets | Metformin
500 mg
tablets | Glucovance
2.5 mg/500 mg
tablets | Glucovance
5 mg/500 mg
tablets |
|---|
| a p<0.001 |
| Mean Final Dose | 20 mg | 1840 mg | 8.8 mg/1760 mg | 17 mg/1740 mg |
| Hemoglobin A1c | N=158 | N=142 | N=154 | N=159 |
| Baseline Mean (%) | 9.63 | 9.51 | 9.43 | 9.44 |
| Final Mean | 9.61 | 9.82 | 7.92 | 7.91 |
| Difference from Glyburide | | | −1.69a | −1.70a |
| Difference from Metformin | | | −1.90a | −1.91a |
| Fasting Plasma Glucose | N=163 | N=152 | N=160 | N=160 |
| Baseline Mean (mg/dL) | 218.4 | 213.4 | 212.2 | 210.2 |
| Final Mean | 221.0 | 233.8 | 169.6 | 161.1 |
| Difference from Glyburide | | | −51.3a | −59.9a |
| Difference from Metformin | | | −64.2a | −72.7a |
Body Weight Mean Change
from Baseline | +0.43 kg | −2.76 kg | +0.75 kg | +0.47 kg |
| Final HbA1c Distribution (%) | N=158 | N=142 | N=154 | N=159 |
| <7% | 2.5% | 2.8% | 24.7% | 22.6% |
| ≥7% and <8% | 9.5% | 11.3% | 33.1% | 37.1% |
| ≥8% | 88% | 85.9% | 42.2% | 40.3% |
After 16 weeks, there was no significant change in the mean HbA1c in patients randomized to glyburide or to metformin therapy. Treatment with Glucovance at doses up to 20 mg/2000 mg per day resulted in significant lowering of HbA1c, FPG, and PPG from baseline compared to glyburide or metformin alone.
Addition of Thiazolidinediones to Glucovance Therapy
In a 24-week, double-blind, multicenter U.S. clinical trial, patients with type 2 diabetes not adequately controlled on current oral antihyperglycemic therapy (either monotherapy or combination therapy) were first switched to open label Glucovance 2.5 mg/500 mg tablets and titrated to a maximum daily dose of 10 mg/2000 mg. A total of 365 patients inadequately controlled (HbA1c >7.0% and ≤10%) after 10 to 12 weeks of a daily Glucovance dose of at least 7.5 mg/1500 mg were randomized to receive add-on therapy with rosiglitazone 4 mg or placebo once daily. After 8 weeks, the rosiglitazone dose was increased to a maximum of 8 mg daily as needed to reach a target mean daily glucose of 126 mg/dL or HbA1c <7%. Trial data at 24 weeks or at the last prior visit are summarized in Table 4.
Table 4: Effects of Adding Rosiglitazone or Placebo in Patients Treated with Glucovance in a 24-Week Trial
| | Placebo
+
Glucovance | Rosiglitazone
+
Glucovance |
|---|
| a Adjusted for the baseline mean difference |
| b p<0.001 |
Mean Final Dose
Glucovance
Rosiglitazone | 10 mg/1992 mg
0 mg | 9.6 mg/1914 mg
7.4 mg |
| Hemoglobin A1c | N=178 | N=177 |
| Baseline Mean (%) | 8.09 | 8.14 |
| Final Mean | 8.21 | 7.23 |
| Difference from Placeboa | | −1.02b |
| Fasting Plasma Glucose | N=181 | N=176 |
| Baseline Mean (mg/dL) | 173.1 | 178.4 |
| Final Mean | 181.4 | 136.3 |
| Difference from Placeboa | | −48.5b |
Body Weight Mean Change
from Baseline | +0.03 kg | + 3.03 kg |
| Final HbA1c Distribution (%) | N=178 | N=177 |
| <7% | 13.5% | 42.4% |
| ≥7% and <8% | 32.0% | 38.4% |
| ≥8% | 54.5% | 19.2% |
For patients who did not achieve adequate glycemic control on Glucovance, the addition of rosiglitazone, compared to placebo, resulted in significant lowering of HbA1c and FPG.
top
Indications and Usage
Glucovance is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
top
Contraindications
Glucovance (Glyburide and Metformin HCl) Tablets is contraindicated in patients with:
- Renal disease or renal dysfunction (e.g., as suggested by serum creatinine levels ≥1.5 mg/dL [males], ≥1.4 mg/dL [females], or abnormal creatinine clearance) which may also result from conditions such as cardiovascular collapse (shock), acute myocardial infarction, and septicemia (see WARNINGS and PRECAUTIONS).
- Known hypersensitivity to metformin hydrochloride or glyburide.
- Acute or chronic metabolic acidosis, including diabetic ketoacidosis, with or without coma. Diabetic ketoacidosis should be treated with insulin.
Glucovance should be temporarily discontinued in patients undergoing radiologic studies involving intravascular administration of iodinated contrast materials, because use of such products may result in acute alteration of renal function. (See also PRECAUTIONS.)
top
Warnings
Metformin Hydrochloride
Lactic acidosis:
Lactic acidosis is a rare, but serious, metabolic complication that can occur due to metformin accumulation during treatment with Glucovance; when it occurs, it is fatal in approximately 50% of cases. Lactic acidosis may also occur in association with a number of pathophysiologic conditions, including diabetes mellitus, and whenever there is significant tissue hypoperfusion and hypoxemia. Lactic acidosis is characterized by elevated blood lactate levels (>5 mmol/L), decreased blood pH, electrolyte disturbances with an increased anion gap, and an increased lactate/pyruvate ratio. When metformin is implicated as the cause of lactic acidosis, metformin plasma levels >5 µg/mL are generally found.
The reported incidence of lactic acidosis in patients receiving metformin hydrochloride is very low (approximately 0.03 cases/1000 patient-years, with approximately 0.015 fatal cases/1000 patient-years). In more than 20,000 patient-years exposure to metformin in clinical trials, there were no reports of lactic acidosis. Reported cases have occurred primarily in diabetic patients with significant renal insufficiency, including both intrinsic renal disease and renal hypoperfusion, often in the setting of multiple concomitant medical/surgical problems and multiple concomitant medications. Patients with congestive heart failure requiring pharmacologic management, in particular those with unstable or acute congestive heart failure who are at risk of hypoperfusion and hypoxemia, are at increased risk of lactic acidosis. The risk of lactic acidosis increases with the degree of renal dysfunction and the patient's age. The risk of lactic acidosis may, therefore, be significantly decreased by regular monitoring of renal function in patients taking metformin and by use of the minimum effective dose of metformin. In particular, treatment of the elderly should be accompanied by careful monitoring of renal function. Glucovance treatment should not be initiated in patients≥80 years of age unless measurement of creatinine clearance demonstrates that renal function is not reduced, as these patients are more susceptible to developing lactic acidosis. In addition, Glucovance should be promptly withheld in the presence of any condition associated with hypoxemia, dehydration, or sepsis. Because impaired hepatic function may significantly limit the ability to clear lactate, Glucovance should generally be avoided in patients with clinical or laboratory evidence of hepatic disease. Patients should be cautioned against excessive alcohol intake, either acute or chronic, when taking Glucovance, since alcohol potentiates the effects of metformin hydrochloride on lactate metabolism. In addition, Glucovance should be temporarily discontinued prior to any intravascular radiocontrast study and for any surgical procedure (see also PRECAUTIONS).
The onset of lactic acidosis often is subtle, and accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, increasing somnolence, and nonspecific abdominal distress. There may be associated hypothermia, hypotension, and resistant bradyarrhythmias with more marked acidosis. The patient and the patient's physician must be aware of the possible importance of such symptoms and the patient should be instructed to notify the physician immediately if they occur (see also PRECAUTIONS). Glucovance should be withdrawn until the situation is clarified. Serum electrolytes, ketones, blood glucose, and if indicated, blood pH, lactate levels, and even blood metformin levels may be useful. Once a patient is stabilized on any dose level of Glucovance, gastrointestinal symptoms, which are common during initiation of therapy with metformin, are unlikely to be drug related. Later occurrence of gastrointestinal symptoms could be due to lactic acidosis or other serious disease.
Levels of fasting venous plasma lactate above the upper limit of normal but less than 5 mmol/L in patients taking Glucovance do not necessarily indicate impending lactic acidosis and may be explainable by other mechanisms, such as poorly controlled diabetes or obesity, vigorous physical activity, or technical problems in sample handling. (See also PRECAUTIONS.)
Lactic acidosis should be suspected in any diabetic patient with metabolic acidosis lacking evidence of ketoacidosis (ketonuria and ketonemia).
Lactic acidosis is a medical emergency that must be treated in a hospital setting. In a patient with lactic acidosis who is taking Glucovance, the drug should be discontinued immediately and general supportive measures promptly instituted. Because metformin hydrochloride is dialyzable (with a clearance of up to 170 mL/min under good hemodynamic conditions), prompt hemodialysis is recommended to correct the acidosis and remove the accumulated metformin. Such management often results in prompt reversal of symptoms and recovery. (See also CONTRAINDICATIONS and PRECAUTIONS.)
SPECIAL WARNING ON INCREASED RISK OF CARDIOVASCULAR MORTALITY
The administration of oral hypoglycemic drugs has been reported to be associated with increased cardiovascular mortality as compared to treatment with diet alone or diet plus insulin. This warning is based on the study conducted by the University Group Diabetes Program (UGDP), a long-term prospective clinical trial designed to evaluate the effectiveness of glucose-lowering drugs in preventing or delaying vascular complications in patients with non-insulin-dependent diabetes. The study involved 823 patients who were randomly assigned to 1 of 4 treatment groups (Diabetes 19 (Suppl. 2):747-830, 1970).
UGDP reported that patients treated for 5 to 8 years with diet plus a fixed dose of tolbutamide (1.5 g per day) had a rate of cardiovascular mortality approximately 2 ½ times that of patients treated with diet alone. A significant increase in total mortality was not observed, but the use of tolbutamide was discontinued based on the increase in cardiovascular mortality, thus limiting the opportunity for the study to show an increase in overall mortality. Despite controversy regarding the interpretation of these results, the findings of the UGDP study provide an adequate basis for this warning. The patient should be informed of the potential risks and benefits of glyburide and of alternative modes of therapy.
Although only 1 drug in the sulfonylurea class (tolbutamide) was included in this study, it is prudent from a safety standpoint to consider that this warning may also apply to other hypoglycemic drugs in this class, in view of their close similarities in mode of action and chemical structure.
top
Precautions
General
Macrovascular Outcomes
There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with Glucovance or any other antidiabetic drug.
Glucovance
Hypoglycemia
Glucovance is capable of producing hypoglycemia or hypoglycemic symptoms, therefore, proper patient selection, dosing, and instructions are important to avoid potential hypoglycemic episodes. The risk of hypoglycemia is increased when caloric intake is deficient, when strenuous exercise is not compensated by caloric supplementation, or during concomitant use with other glucose-lowering agents or ethanol. Renal or hepatic insufficiency may cause elevated drug levels of both glyburide and metformin hydrochloride and the hepatic insufficiency may also diminish gluconeogenic capacity, both of which increase the risk of hypoglycemic reactions. Elderly, debilitated, or malnourished patients and those with adrenal or pituitary insufficiency or alcohol intoxication are particularly susceptible to hypoglycemic effects. Hypoglycemia may be difficult to recognize in the elderly, and in people who are taking beta-adrenergic blocking drugs.
Glyburide
Hemolytic anemia
Treatment of patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency with sulfonylurea agents can lead to hemolytic anemia. Because Glucovance belongs to the class of sulfonylurea agents, caution should be used in patients with G6PD deficiency and a non-sulfonylurea alternative should be considered. In postmarketing reports, hemolytic anemia has also been reported in patients who did not have known G6PD deficiency.
Metformin Hydrochloride
Monitoring of renal function
Metformin is known to be substantially excreted by the kidney, and the risk of metformin accumulation and lactic acidosis increases with the degree of impairment of renal function. Thus, patients with serum creatinine levels above the upper limit of normal for their age should not receive Glucovance. In patients with advanced age, Glucovance should be carefully titrated to establish the minimum dose for adequate glycemic effect, because aging is associated with reduced renal function. In elderly patients, particularly those ≥80 years of age, renal function should be monitored regularly and, generally, Glucovance should not be titrated to the maximum dose (see WARNINGS and DOSAGE AND ADMINISTRATION). Before initiation of Glucovance therapy and at least annually thereafter, renal function should be assessed and verified as normal. In patients in whom development of renal dysfunction is anticipated, renal function should be assessed more frequently and Glucovance discontinued if evidence of renal impairment is present.
Use of concomitant medications that may affect renal function or metformin disposition
Concomitant medication(s) that may affect renal function or result in significant hemodynamic change or may interfere with the disposition of metformin, such as cationic drugs that are eliminated by renal tubular secretion (see PRECAUTIONS: Drug Interactions), should be used with caution.
Radiologic studies involving the use of intravascular iodinated contrast materials (for example, intravenous urogram, intravenous cholangiography, angiography, and computed tomography (CT) scans with intravascular contrast materials)
Intravascular contrast studies with iodinated materials can lead to acute alteration of renal function and have been associated with lactic acidosis in patients receiving metformin (see CONTRAINDICATIONS). Therefore, in patients in whom any such study is planned, Glucovance should be temporarily discontinued at the time of or prior to the procedure, and withheld for 48 hours subsequent to the procedure and reinstituted only after renal function has been reevaluated and found to be normal.
Hypoxic states
Cardiovascular collapse (shock) from whatever cause, acute congestive heart failure, acute myocardial infarction, and other conditions characterized by hypoxemia have been associated with lactic acidosis and may also cause prerenal azotemia. When such events occur in patients on Glucovance therapy, the drug should be promptly discontinued.
Surgical procedures
Glucovance therapy should be temporarily suspended for any surgical procedure (except minor procedures not associated with restricted intake of food and fluids) and should not be restarted until the patient's oral intake has resumed and renal function has been evaluated as normal.
Alcohol intake
Alcohol is known to potentiate the effect of metformin on lactate metabolism. Patients, therefore, should be warned against excessive alcohol intake, acute or chronic, while receiving Glucovance. Due to its effect on the gluconeogenic capacity of the liver, alcohol may also increase the risk of hypoglycemia.
Impaired hepatic function
Since impaired hepatic function has been associated with some cases of lactic acidosis, Glucovance should generally be avoided in patients with clinical or laboratory evidence of hepatic disease.
Vitamin B12 levels
In controlled clinical trials with metformin of 29 weeks duration, a decrease to subnormal levels of previously normal serum vitamin B12, without clinical manifestations, was observed in approximately 7% of patients. Such decrease, possibly due to interference with B12 absorption from the B12-intrinsic factor complex, is, however, very rarely associated with anemia and appears to be rapidly reversible with discontinuation of metformin or vitamin B12 supplementation. Measurement of hematologic parameters on an annual basis is advised in patients on metformin and any apparent abnormalities should be appropriately investigated and managed (see PRECAUTIONS: Laboratory Tests).
Certain individuals (those with inadequate vitamin B12 or calcium intake or absorption) appear to be predisposed to developing subnormal vitamin B12 levels. In these patients, routine serum vitamin B12 measurements at 2- to 3-year intervals may be useful.
Change in clinical status of patients with previously controlled type 2 diabetes
A patient with type 2 diabetes previously well controlled on metformin who develops laboratory abnormalities or clinical illness (especially vague and poorly defined illness) should be evaluated promptly for evidence of ketoacidosis or lactic acidosis. Evaluation should include serum electrolytes and ketones, blood glucose and, if indicated, blood pH, lactate, pyruvate, and metformin levels. If acidosis of either form occurs, Glucovance must be stopped immediately and other appropriate corrective measures initiated (see also WARNINGS).
Addition of Thiazolidinediones to Glucovance Therapy
Hypoglycemia
Patients receiving Glucovance in combination with a thiazolidinedione may be at risk for hypoglycemia.
Weight gain
Weight gain was seen with the addition of rosiglitazone to Glucovance, similar to that reported for thiazolidinedione therapy alone.
Hepatic effects
When a thiazolidinedione is used in combination with Glucovance, periodic monitoring of liver function tests should be performed in compliance with the labeled recommendations for the thiazolidinedione.
Information for Patients
Glucovance
Patients should be informed of the potential risks and benefits of Glucovance and of alternative modes of therapy. They should also be informed about the importance of adherence to dietary instructions, of a regular exercise program, and of regular testing of blood glucose, glycosylated hemoglobin, renal function, and hematologic parameters.
The risks of lactic acidosis associated with metformin therapy, its symptoms, and conditions that predispose to its development, as noted in the WARNINGS and PRECAUTIONS sections, should be explained to patients. Patients should be advised to discontinue Glucovance immediately and to promptly notify their health practitioner if unexplained hyperventilation, myalgia, malaise, unusual somnolence, or other nonspecific symptoms occur. Once a patient is stabilized on any dose level of Glucovance, gastrointestinal symptoms, which are common during initiation of metformin therapy, are unlikely to be drug related. Later occurrence of gastrointestinal symptoms could be due to lactic acidosis or other serious disease.
The risks of hypoglycemia, its symptoms and treatment, and conditions that predispose to its development should be explained to patients and responsible family members.
Patients should be counseled against excessive alcohol intake, either acute or chronic, while receiving Glucovance.
Laboratory Tests
Periodic fasting blood glucose and glycosylated hemoglobin (HbA1c) measurements should be performed to monitor therapeutic response.
Initial and periodic monitoring of hematologic parameters (e.g., hemoglobin/hematocrit and red blood cell indices) and renal function (serum creatinine) should be performed, at least on an annual basis. While megaloblastic anemia has rarely been seen with metformin therapy, if this is suspected, vitamin B12 deficiency should be excluded.
Drug Interactions
Glucovance
Certain drugs tend to produce hyperglycemia and may lead to loss of blood glucose control. These drugs include the thiazides and other diuretics, corticosteroids, phenothiazines, thyroid products, estrogens, oral contraceptives, phenytoin, nicotinic acid, sympathomimetics, calcium channel blocking drugs, and isoniazid. When such drugs are administered to a patient receiving Glucovance, the patient should be closely observed for loss of blood glucose control. When such drugs are withdrawn from a patient receiving Glucovance, the patient should be observed closely for hypoglycemia. Metformin is negligibly bound to plasma proteins and is, therefore, less likely to interact with highly protein-bound drugs such as salicylates, sulfonamides, chloramphenicol, and probenecid as compared to sulfonylureas, which are extensively bound to serum proteins.
Glyburide
The hypoglycemic action of sulfonylureas may be potentiated by certain drugs including nonsteroidal anti-inflammatory agents and other drugs that are highly protein bound, salicylates, sulfonamides, chloramphenicol, probenecid, coumarins, monoamine oxidase inhibitors, and beta adrenergic blocking agents. When such drugs are administered to a patient receiving Glucovance, the patient should be observed closely for hypoglycemia. When such drugs are withdrawn from a patient receiving Glucovance, the patient should be observed closely for loss of blood glucose control.
A possible interaction between glyburide and ciprofloxacin, a fluoroquinolone antibiotic, has been reported, resulting in a potentiation of the hypoglycemic action of glyburide. The mechanism for this interaction is not known.
A potential interaction between oral miconazole and oral hypoglycemic agents leading to severe hypoglycemia has been reported. Whether this interaction also occurs with the intravenous, topical, or vaginal preparations of miconazole is not known.
Metformin Hydrochloride
Furosemide
A single-dose, metformin-furosemide drug interaction study in healthy subjects demonstrated that pharmacokinetic parameters of both compounds were affected by coadministration. Furosemide increased the metformin plasma and blood Cmax by 22% and blood AUC by 15%, without any significant change in metformin renal clearance. When administered with metformin, the Cmax and AUC of furosemide were 31% and 12% smaller, respectively, than when administered alone, and the terminal half-life was decreased by 32%, without any significant change in furosemide renal clearance. No information is available about the interaction of metformin and furosemide when coadministered chronically.
Nifedipine
A single-dose, metformin-nifedipine drug interaction study in normal healthy volunteers demonstrated that coadministration of nifedipine increased plasma metformin Cmax and AUC by 20% and 9%, respectively, and increased the amount excreted in the urine. Tmax and half-life were unaffected. Nifedipine appears to enhance the absorption of metformin. Metformin had minimal effects on nifedipine.
Cationic drugs
Cationic drugs (e.g., amiloride, digoxin, morphine, procainamide, quinidine, quinine, ranitidine, triamterene, trimethoprim, or vancomycin) that are eliminated by renal tubular secretion theoretically have the potential for interaction with metformin by competing for common renal tubular transport systems. Such interaction between metformin and oral cimetidine has been observed in normal healthy volunteers in both single- and multiple-dose, metformin-cimetidine drug interaction studies, with a 60% increase in peak metformin plasma and whole blood concentrations and a 40% increase in plasma and whole blood metformin AUC. There was no change in elimination half-life in the single-dose study. Metformin had no effect on cimetidine pharmacokinetics. Although such interactions remain theoretical (except for cimetidine), careful patient monitoring and dose adjustment of Glucovance and/or the interfering drug is recommended in patients who are taking cationic medications that are excreted via the proximal renal tubular secretory system.
Other
In healthy volunteers, the pharmacokinetics of metformin and propranolol and metformin and ibuprofen were not affected when coadministered in single-dose interaction studies.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No animal studies have been conducted with the combined products in Glucovance. The following data are based on findings in studies performed with the individual products.
Glyburide
Studies in rats with glyburide alone at doses up to 300 mg/kg/day (approximately 145 times the maximum recommended human daily dose of 20 mg for the glyburide component of Glucovance based on body surface area comparisons) for 18 months revealed no carcinogenic effects. In a 2-year oncogenicity study of glyburide in mice, there was no evidence of treatment-related tumors.
There was no evidence of mutagenic potential of glyburide alone in the following in vitro tests: Salmonella microsome test (Ames test) and in the DNA damage/alkaline elution assay.
Metformin Hydrochloride
Long-term carcinogenicity studies were performed with metformin alone in rats (dosing duration of 104 weeks) and mice (dosing duration of 91 weeks) at doses up to and including 900 mg/kg/day and 1500 mg/kg/day, respectively. These doses are both approximately 4 times the maximum recommended human daily dose of 2000 mg of the metformin component of Glucovance based on body surface area comparisons. No evidence of carcinogenicity with metformin alone was found in either male or female mice. Similarly, there was no tumorigenic potential observed with metformin alone in male rats. There was, however, an increased incidence of benign stromal uterine polyps in female rats treated with 900 mg/kg/day of metformin alone.
There was no evidence of a mutagenic potential of metformin alone in the following in vitro tests: Ames test (S. typhimurium), gene mutation test (mouse lymphoma cells), or chromosomal aberrations test (human lymphocytes). Results in the in vivo mouse micronucleus test were also negative.
Fertility of male or female rats was unaffected by metformin alone when administered at doses as high as 600 mg/kg/day, which is approximately 3 times the maximum recommended human daily dose of the metformin component of Glucovance based on body surface area comparisons.
Pregnancy
Teratogenic Effects: Pregnancy Category B
Recent information strongly suggests that abnormal blood glucose levels during pregnancy are associated with a higher incidence of congenital abnormalities. Most experts recommend that insulin be used during pregnancy to maintain blood glucose as close to normal as possible. Because animal reproduction studies are not always predictive of human response, Glucovance should not be used during pregnancy unless clearly needed. (See below.)
There are no adequate and well-controlled studies in pregnant women with Glucovance or its individual components. No animal studies have been conducted with the combined products in Glucovance. The following data are based on findings in studies performed with the individual products.
Glyburide
Reproduction studies were performed in rats and rabbits at doses up to 500 times the maximum recommended human daily dose of 20 mg of the glyburide component of Glucovance based on body surface area comparisons and revealed no evidence of impaired fertility or harm to the fetus due to glyburide.
Metformin Hydrochloride
Metformin alone was not teratogenic in rats or rabbits at doses up to 600 mg/kg/day. This represents an exposure of about 2 and 6 times the maximum recommended human daily dose of 2000 mg of the metformin component of Glucovance based on body surface area comparisons for rats and rabbits, respectively. Determination of fetal concentrations demonstrated a partial placental barrier to metformin.
Nonteratogenic Effects
Prolonged severe hypoglycemia (4 to 10 days) has been reported in neonates born to mothers who were receiving a sulfonylurea drug at the time of delivery. This has been reported more frequently with the use of agents with prolonged half-lives. It is not recommended that Glucovance be used during pregnancy. However, if it is used, Glucovance should be discontinued at least 2 weeks before the expected delivery date. (See Pregnancy: Teratogenic Effects: Pregnancy Category B.)
Nursing Mothers
Although it is not known whether glyburide is excreted in human milk, some sulfonylurea drugs are known to be excreted in human milk. Studies in lactating rats show that metformin is excreted into milk and reaches levels comparable to those in plasma. Similar studies have not been conducted in nursing mothers. Because the potential for hypoglycemia in nursing infants may exist, a decision should be made whether to discontinue nursing or to discontinue Glucovance, taking into account the importance of the drug to the mother. If Glucovance is discontinued, and if diet alone is inadequate for controlling blood glucose, insulin therapy should be considered.
Pediatric Use
The safety and efficacy of Glucovance were evaluated in an active-controlled, double-blind, 26-week randomized trial involving a total of 167 pediatric patients (ranging from 9 to 16 years of age) with type 2 diabetes. Glucovance was not shown statistically to be superior to either metformin or glyburide with respect to reducing HbA1c from baseline (see Table 5). No unexpected safety findings were associated with Glucovance in this trial.
Table 5: HbA1c (Percent) Change From Baseline at 26 Weeks: Pediatric Study
| | Glyburide
2.5 mg
tablets | Metformin
500 mg
tablets | Glucovance
1.25 mg/250 mg
tablets |
|---|
| Mean Final Dose | 6.5 mg | 1500 mg | 3.1 mg/623 mg |
| Hemoglobin A1c | N=49 | N=54 | N=57 |
| Baseline Mean (%) | 7.70 | 7.99 | 7.85 |
| Mean Change from Baseline | −0.96 | −0.48 | −0.80 |
| Difference from Metformin | | | −0.32 |
| Difference from Glyburide | | | +0.16 |
Geriatric Use
Of the 642 patients who received Glucovance in double-blind clinical studies, 23.8% were 65 and older while 2.8% were 75 and older. Of the 1302 patients who received Glucovance in open-label clinical studies, 20.7% were 65 and older while 2.5% were 75 and older. No overall differences in effectiveness or safety were observed between these patients and younger patients, and other reported clinical experience has not identified differences in response between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Metformin hydrochloride is known to be substantially excreted by the kidney and because the risk of serious adverse reactions to the drug is greater in patients with impaired renal function, Glucovance should only be used in patients with normal renal function (see CONTRAINDICATIONS, WARNINGS, and CLINICAL PHARMACOLOGY: Pharmacokinetics). Because aging is associated with reduced renal function, Glucovance should be used with caution as age increases. Care should be taken in dose selection and should be based on careful and regular monitoring of renal function. Generally, elderly patients should not be titrated to the maximum dose of Glucovance (see also WARNINGS and DOSAGE AND ADMINISTRATION).
top
Adverse Reactions
Glucovance
In double-blind clinical trials involving Glucovance as initial therapy or as second-line therapy, a total of 642 patients received Glucovance, 312 received metformin therapy, 324 received glyburide therapy, and 161 received placebo. The percent of patients reporting events and types of adverse events reported in clinical trials of Glucovance (all strengths) as initial therapy and second-line therapy are listed in Table 6.
Table 6: Most Common Clinical Adverse Events (>5%) in Double-Blind Clinical Studies of Glucovance Used as Initial or Second-Line Therapy
Adverse Event | Number (%) of Patients |
Placebo
N=161 | Glyburide
N=324 | Metformin
N=312 | Glucovance
N=642 |
| Upper respiratory infection | 22 (13.7) | 57 (17.6) | 51 (16.3) | 111 (17.3) |
| Diarrhea | 9 (5.6) | 20 (6.2) | 64 (20.5) | 109 (17.0) |
| Headache | 17 (10.6) | 37 (11.4) | 29 (9.3) | 57 (8.9) |
| Nausea/vomiting | 10 (6.2) | 17 (5.2) | 38 (12.2) | 49 (7.6) |
| Abdominal pain | 6 (3.7) | 10 (3.1) | 25 (8.0) | 44 (6.9) |
| Dizziness | 7 (4.3) | 18 (5.6) | 12 (3.8) | 35 (5.5) |
In a controlled clinical trial of rosiglitazone versus placebo in patients treated with Glucovance (n=365), 181 patients received Glucovance with rosiglitazone and 184 received Glucovance with placebo.
Edema was reported in 7.7% (14/181) of patients treated with rosiglitazone compared to 2.2% (4/184) of patients treated with placebo. A mean weight gain of 3 kg was observed in rosiglitazone-treated patients.
Disulfiram-like reactions have very rarely been reported in patients treated with glyburide tablets.
Hypoglycemia
In controlled clinical trials of Glucovance there were no hypoglycemic episodes requiring medical intervention and/or pharmacologic therapy; all events were managed by the patients. The incidence of reported symptoms of hypoglycemia (such as dizziness, shakiness, sweating, and hunger), in the initial therapy trial of Glucovance are summarized in Table 7. The frequency of hypoglycemic symptoms in patients treated with Glucovance 1.25 mg/250 mg was highest in patients with a baseline HbA1c 8%. For patients with a baseline HbA1c between 8% and 11% treated with Glucovance 2.5 mg/500 mg as initial therapy, the frequency of hypoglycemic symptoms was 30% to 35%. As second-line therapy in patients inadequately controlled on sulfonylurea alone, approximately 6.8% of all patients treated with Glucovance experienced hypoglycemic symptoms. When rosiglitazone was added to Glucovance therapy, 22% of patients reported 1 or more fingerstick glucose measurements ≤50 mg/dL compared to 3.3% of placebo-treated patients. All hypoglycemic events were managed by the patients and only 1 patient discontinued for hypoglycemia. (See PRECAUTIONS: General: Addition of Thiazolidinediones to Glucovance Therapy.)
Gastrointestinal Reactions
The incidence of GI side effects (diarrhea, nausea/vomiting, and abdominal pain) in the initial therapy trial are summarized in Table 7. Across all Glucovance trials, GI symptoms were the most common adverse events with Glucovance and were more frequent at higher dose levels. In controlled trials, <2% of patients discontinued Glucovance therapy due to GI adverse events.
Table 7: Treatment Emergent Symptoms of Hypoglycemia or Gastrointestinal Adverse Events in a Placebo- and Active-Controlled Trial of Glucovance as Initial Therapy
| Variable | Placebo
N=161 | Glyburide
Tablets
N=160 | Metformin
Tablets
N=159 | Glucovance
1.25 mg/250 mg
Tablets
N=158 | Glucovance
2.5 mg/500 mg
Tablets
N=162 |
| Mean Final Dose | 0 mg | 5.3 mg | 1317 mg | 2.78 mg/557 mg | 4.1 mg/824 mg |
Number (%) of patients
with symptoms of
hypoglycemia | 5 (3.1) | 34 (21.3) | 5 (3.1) | 18 (11.4) | 61 (37.7) |
Number (%) of patients
with gastrointestinal
adverse events | 39 (24.2) | 38 (23.8) | 69 (43.3) | 50 (31.6) | 62 (38.3) |
top
Overdosage
Glyburide
Overdosage of sulfonylureas, including glyburide tablets, can produce hypoglycemia. Mild hypoglycemic symptoms, without loss of consciousness or neurological findings, should be treated aggressively with oral glucose and adjustments in drug dosage and/or meal patterns. Close monitoring should continue until the physician is assured that the patient is out of danger. Severe hypoglycemic reactions with coma, seizure, or other neurological impairment occur infrequently, but constitute medical emergencies requiring immediate hospitalization. If hypoglycemic coma is diagnosed or suspected, the patient should be given a rapid intravenous injection of concentrated (50%) glucose solution. This should be followed by a continuous infusion of a more dilute (10%) glucose solution at a rate that will maintain the blood glucose at a level above 100 mg/dL. Patients should be closely monitored for a minimum of 24 to 48 hours, since hypoglycemia may recur after apparent clinical recovery.
Metformin Hydrochloride
Overdose of metformin hydrochloride has occurred, including ingestion of amounts greater than 50 grams. Hypoglycemia was reported in approximately 10% of cases, but no causal association with metformin hydrochloride has been established. Lactic acidosis has been reported in approximately 32% of metformin overdose cases (see WARNINGS). Metformin is dialyzable with a clearance of up to 170 mL/min under good hemodynamic conditions. Therefore, hemodialysis may be useful for removal of accumulated drug from patients in whom metformin overdosage is suspected.
top
Dosage and Administration
General Considerations
Dosage of Glucovance must be individualized on the basis of both effectiveness and tolerance while not exceeding the maximum recommended daily dose of 20 mg glyburide/2000 mg metformin. Glucovance should be given with meals and should be initiated at a low dose, with gradual dose escalation as described below, in order to avoid hypoglycemia (largely due to glyburide), to reduce GI side effects (largely due to metformin), and to permit determination of the minimum effective dose for adequate control of blood glucose for the individual patient.
With initial treatment and during dose titration, appropriate blood glucose monitoring should be used to determine the therapeutic response to Glucovance and to identify the minimum effective dose for the patient. Thereafter, HbA1c should be measured at intervals of approximately 3 months to assess the effectiveness of therapy. The therapeutic goal in all patients with type 2 diabetes is to decrease FPG, PPG, and HbA1c to normal or as near normal as possible. Ideally, the response to therapy should be evaluated using HbA1c (glycosylated hemoglobin), which is a better indicator of long-term glycemic control than FPG alone.
No studies have been performed specifically examining the safety and efficacy of switching to Glucovance therapy in patients taking concomitant glyburide (or other sulfonylurea) plus metformin. Changes in glycemic control may occur in such patients, with either hyperglycemia or hypoglycemia possible. Any change in therapy of type 2 diabetes should be undertaken with care and appropriate monitoring.
Glucovance in Patients with Inadequate Glycemic Control on Diet and Exercise
Recommended starting dose: 1.25 mg/250 mg once or twice daily with meals.
For patients with type 2 diabetes whose hyperglycemia cannot be satisfactorily managed with diet and exercise alone, the recommended starting dose of Glucovance is 1.25 mg/250 mg once a day with a meal. As initial therapy in patients with baseline HbA1c >9% or an FPG >200 mg/dL, a starting dose of Glucovance 1.25 mg/250 mg twice daily with the morning and evening meals may be used. Dosage increases should be made in increments of 1.25 mg/250 mg per day every 2 weeks up to the minimum effective dose necessary to achieve adequate control of blood glucose. In clinical trials of Glucovance as initial therapy, there was no experience with total daily doses greater than 10 mg/2000 mg per day. Glucovance 5 mg/500 mg should not be used as initial therapy due to an increased risk of hypoglycemia.
Glucovance Use in Patients with Inadequate Glycemic Control on a Sulfonylurea and/or Metformin
Recommended starting dose: 2.5 mg/500 mg or 5 mg/500 mg twice daily with meals.
For patients not adequately controlled on either glyburide (or another sulfonylurea) or metformin alone, the recommended starting dose of Glucovance is 2.5 mg/500 mg or 5 mg/500 mg twice daily with the morning and evening meals. In order to avoid hypoglycemia, the starting dose of Glucovance should not exceed the daily doses of glyburide or metformin already being taken. The daily dose should be titrated in increments of no more than 5 mg/500 mg up to the minimum effective dose to achieve adequate control of blood glucose or to a maximum dose of 20 mg/2000 mg per day.
For patients previously treated with combination therapy of glyburide (or another sulfonylurea) plus metformin, if switched to Glucovance, the starting dose should not exceed the daily dose of glyburide (or equivalent dose of another sulfonylurea) and metformin already being taken. Patients should be monitored closely for signs and symptoms of hypoglycemia following such a switch and the dose of Glucovance should be titrated as described above to achieve adequate control of blood glucose.
Addition of Thiazolidinediones to Glucovance Therapy
For patients not adequately controlled on Glucovance, a thiazolidinedione can be added to Glucovance therapy. When a thiazolidinedione is added to Glucovance therapy, the current dose of Glucovance can be continued and the thiazolidinedione initiated at its recommended starting dose. For patients needing additional glycemic control, the dose of the thiazolidinedione can be increased based on its recommended titration schedule. The increased glycemic control attainable with Glucovance plus a thiazolidinedione may increase the potential for hypoglycemia at any time of day. In patients who develop hypoglycemia when receiving Glucovance and a thiazolidinedione, consideration should be given to reducing the dose of the glyburide component of Glucovance. As clinically warranted, adjustment of the dosages of the other components of the antidiabetic regimen should also be considered.
Specific Patient Populations
Glucovance is not recommended for use during pregnancy. The initial and maintenance dosing of Glucovance should be conservative in patients with advanced age, due to the potential for decreased renal function in this population. Any dosage adjustment requires a careful assessment of renal function. Generally, elderly, debilitated, and malnourished patients should not be titrated to the maximum dose of Glucovance to avoid the risk of hypoglycemia. Monitoring of renal function is necessary to aid in prevention of metformin-associated lactic acidosis, particularly in the elderly. (See WARNINGS.)
top
How Supplied
Glucovance® (Glyburide and Metformin HCl) Tablets
Glucovance 1.25 mg/250 mg tablet is a pale yellow, capsule-shaped, bevel-edged, biconvex, film-coated tablet with "BMS" debossed on one side and "6072" debossed on the opposite side.
Glucovance 2.5 mg/500 mg tablet is a pale orange, capsule-shaped, bevel-edged, biconvex, film-coated tablet with "BMS" debossed on one side and "6073" debossed on the opposite side.
Glucovance 5 mg/500 mg tablet is a yellow, capsule-shaped, bevel-edged, biconvex, film-coated tablet with "BMS" debossed on one side and "6074" debossed on the opposite side.
| Glucovance | NDC 0087-xxxx-xx for unit of use |
Glyburide
(mg) | Metformin hydrochloride
(mg) | Bottle of 100 |
| 1.25 | 250 | 6072-11 |
| 2.5 | 500 | 6073-11 |
| 5 | 500 | 6074-11 |
STORAGE
Store at temperatures up to 25°C (77°F). [See USP Controlled Room Temperature.]
Dispense in light-resistant containers.
Glucovance® is a registered trademark of Merck Santé S.A.S., an associate of Merck KGaA of Darmstadt, Germany. Licensed to Bristol-Myers Squibb Company.
GLUCOPHAGE® is a registered trademark of Merck Santé S.A.S., an associate of Merck KGaA of Darmstadt, Germany. Licensed to Bristol-Myers Squibb Company.
Micronase® is a registered trademark of Pharmacia & Upjohn Company.
Distributed by:
Bristol-Myers Squibb Company
Princeton, NJ 08543 USA
last updated 02/2009
Glucovance patient information (in plain English)
Detailed Info on Signs, Symptoms, Causes, Treatments of Diabetes
The information in this monograph is not intended to cover all possible uses, directions, precautions, drug interactions or adverse effects. This information is generalized and is not intended as specific medical advice. If you have questions about the medicines you are taking or would like more information, check with your doctor, pharmacist, or nurse.
back to: Browse all Medications for Diabetes