Brand Name: Janumet
Generic Name: Sitagliptin and Metformin Hydrochloride
Contents:
Indications and Usage
Dosage and Administration
Dosage Forms and Strengths
Contraindications
Warnings and Precautions
Adverse Reactions
Drug Interactions
Use in Specific Populations
Overdose
Description
Pharmacology
Nonclinical Toxicology
Clinical Studies
How Supplied
Patient Counseling Information
Janumet, Sitagliptin and Metformin Hydrochloride, Patient Information (in plain English)
WARNING: LACTIC ACIDOSIS
Lactic acidosis is a rare, but serious complication that can occur due to metformin accumulation. The risk increases with conditions such as sepsis, dehydration, excess alcohol intake, hepatic insufficiency, renal impairment, and acute congestive heart failure.
The onset is often subtle, accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, increasing somnolence, and nonspecific abdominal distress. Laboratory abnormalities include low pH, increased anion gap and elevated blood lactate.
If acidosis is suspected, Janumet1 should be discontinued and the patient hospitalized immediately. [See Warnings and Precautions]
Indications and Usage
Janumet is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus when treatment with both sitagliptin and metformin is appropriate. [See Clinical Studies.]
Important Limitations of Use
Janumet should not be used in patients with type 1 diabetes or for the treatment of diabetic ketoacidosis, as it would not be effective in these settings.
Janumet has not been studied in combination with insulin.
top
Dosage and Administration
Recommended Dosing
The dosage of antihyperglycemic therapy with Janumet should be individualized on the basis of the patient's current regimen, effectiveness, and tolerability while not exceeding the maximum recommended daily dose of 100 mg sitagliptin and 2000 mg metformin. Initial combination therapy or maintenance of combination therapy should be individualized and left to the discretion of the health care provider.
Janumet should generally be given twice daily with meals, with gradual dose escalation, to reduce the gastrointestinal (GI) side effects due to metformin.
The starting dose of Janumet should be based on the patient's current regimen. Janumet should be given twice daily with meals. The following doses are available:
50 mg sitagliptin/500 mg metformin hydrochloride
50 mg sitagliptin/1000 mg metformin hydrochloride.
Patients inadequately controlled with diet and exercise alone
If therapy with a combination tablet containing sitagliptin and metformin is considered appropriate for a patient with type 2 diabetes mellitus inadequately controlled with diet and exercise alone, the recommended starting dose is 50 mg sitagliptin/500 mg metformin hydrochloride twice daily. Patients with inadequate glycemic control on this dose can be titrated up to 50 mg sitagliptin/1000 mg metformin hydrochloride twice daily.
Patients inadequately controlled on metformin monotherapy
If therapy with a combination tablet containing sitagliptin and metformin is considered appropriate for a patient inadequately controlled on metformin alone, the recommended starting dose of Janumet should provide sitagliptin dosed as 50 mg twice daily (100 mg total daily dose) and the dose of metformin already being taken. For patients taking metformin 850 mg twice daily, the recommended starting dose of Janumet is 50 mg sitagliptin/1000 mg metformin hydrochloride twice daily.
Patients inadequately controlled on sitagliptin monotherapy
If therapy with a combination tablet containing sitagliptin and metformin is considered appropriate for a patient inadequately controlled on sitagliptin alone, the recommended starting dose of Janumet is 50 mg sitagliptin/500 mg metformin hydrochloride twice daily. Patients with inadequate control on this dose can be titrated up to 50 mg sitagliptin/1000 mg metformin hydrochloride twice daily. Patients taking sitagliptin monotherapy dose-adjusted for renal insufficiency should not be switched to Janumet [see Contraindications].
Patients switching from co-administration of sitagliptin and metformin
For patients switching from sitagliptin co-administrated with metformin, Janumet may be initiated at the dose of sitagliptin and metformin already being taken.
Patients inadequately controlled on dual combination therapy with any two of the following antihyperglycemic agents: sitagliptin, metformin or a sulfonylurea
If therapy with a combination tablet containing sitagliptin and metformin is considered appropriate in this setting, the usual starting dose of Janumet should provide sitagliptin dosed as 50 mg twice daily (100 mg total daily dose). In determining the starting dose of the metformin component, the patient's level of glycemic control and current dose (if any) of metformin should be considered. Gradual dose escalation to reduce the gastrointestinal (GI) side effects associated with metformin should be considered. Patients currently on or initiating a sulfonylurea may require lower sulfonylurea doses to reduce the risk of hypoglycemia [see Warnings and Precautions].
No studies have been performed specifically examining the safety and efficacy of Janumet in patients previously treated with other oral antihyperglycemic agents and switched to Janumet. Any change in therapy of type 2 diabetes should be undertaken with care and appropriate monitoring as changes in glycemic control can occur.
top
Dosage Forms and Strengths
- 50 mg/500 mg tablets are light pink, capsule-shaped, film-coated tablets with "575" debossed on one side.
- 50 mg/1000 mg tablets are red, capsule-shaped, film-coated tablets with "577" debossed on one side.
top
Contraindications
Janumet (sitagliptin/metformin HCl) is contraindicated in patients with:
- Renal disease or renal dysfunction, e.g., as suggested by serum creatinine levels ≥1.5 mg/dL [males], ≥1.4 mg/dL [females] or abnormal creatinine clearance which may also result from conditions such as cardiovascular collapse (shock), acute myocardial infarction, and septicemia [see Warnings and Precautions].
- Acute or chronic metabolic acidosis, including diabetic ketoacidosis, with or without coma.
- History of a serious hypersensitivity reaction to Janumet or sitagliptin (one of the components of Janumet), such as anaphylaxis or angioedema. [See Warnings and Precautions and Adverse Reactions.]
Janumet should be temporarily discontinued in patients undergoing radiologic studies involving intravascular administration of iodinated contrast materials, because use of such products may result in acute alteration of renal function [see Warnings and Precautions].
top
Warnings and Precautions
Lactic Acidosis
Metformin hydrochloride
Lactic acidosis is a rare, but serious, metabolic complication that can occur due to metformin accumulation during treatment with Janumet; when it occurs, it is fatal in approximately 50% of cases. Lactic acidosis may also occur in association with a number of pathophysiologic conditions, including diabetes mellitus, and whenever there is significant tissue hypoperfusion and hypoxemia. Lactic acidosis is characterized by elevated blood lactate levels (>5 mmol/L), decreased blood pH, electrolyte disturbances with an increased anion gap, and an increased lactate/pyruvate ratio. When metformin is implicated as the cause of lactic acidosis, metformin plasma levels >5 μg/mL are generally found.
The reported incidence of lactic acidosis in patients receiving metformin hydrochloride is very low (approximately 0.03 cases/1000 patient-years, with approximately 0.015 fatal cases/1000 patient-years). In more than 20,000 patient-years exposure to metformin in clinical trials, there were no reports of lactic acidosis. Reported cases have occurred primarily in diabetic patients with significant renal insufficiency, including both intrinsic renal disease and renal hypoperfusion, often in the setting of multiple concomitant medical/surgical problems and multiple concomitant medications. Patients with congestive heart failure requiring pharmacologic management, in particular those with unstable or acute congestive heart failure who are at risk of hypoperfusion and hypoxemia, are at increased risk of lactic acidosis. The risk of lactic acidosis increases with the degree of renal dysfunction and the patient's age. The risk of lactic acidosis may, therefore, be significantly decreased by regular monitoring of renal function in patients taking metformin and by use of the minimum effective dose of metformin. In particular, treatment of the elderly should be accompanied by careful monitoring of renal function. Metformin treatment should not be initiated in patients ≥80 years of age unless measurement of creatinine clearance demonstrates that renal function is not reduced, as these patients are more susceptible to developing lactic acidosis. In addition, metformin should be promptly withheld in the presence of any condition associated with hypoxemia, dehydration, or sepsis. Because impaired hepatic function may significantly limit the ability to clear lactate, metformin should generally be avoided in patients with clinical or laboratory evidence of hepatic disease. Patients should be cautioned against excessive alcohol intake, either acute or chronic, when taking metformin, since alcohol potentiates the effects of metformin hydrochloride on lactate metabolism. In addition, metformin should be temporarily discontinued prior to any intravascular radiocontrast study and for any surgical procedure [see Warnings and Precautions].
The onset of lactic acidosis often is subtle, and accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, increasing somnolence, and nonspecific abdominal distress. There may be associated hypothermia, hypotension, and resistant bradyarrhythmias with more marked acidosis. The patient and the patient's physician must be aware of the possible importance of such symptoms and the patient should be instructed to notify the physician immediately if they occur [see Warnings and Precautions]. Metformin should be withdrawn until the situation is clarified. Serum electrolytes, ketones, blood glucose, and if indicated, blood pH, lactate levels, and even blood metformin levels may be useful. Once a patient is stabilized on any dose level of metformin, gastrointestinal symptoms, which are common during initiation of therapy, are unlikely to be drug related. Later occurrence of gastrointestinal symptoms could be due to lactic acidosis or other serious disease.
Levels of fasting venous plasma lactate above the upper limit of normal but less than 5 mmol/L in patients taking metformin do not necessarily indicate impending lactic acidosis and may be explainable by other mechanisms, such as poorly controlled diabetes or obesity, vigorous physical activity, or technical problems in sample handling [see Warnings and Precautions].
Lactic acidosis should be suspected in any diabetic patient with metabolic acidosis lacking evidence of ketoacidosis (ketonuria and ketonemia).
Lactic acidosis is a medical emergency that must be treated in a hospital setting. In a patient with lactic acidosis who is taking metformin, the drug should be discontinued immediately and general supportive measures promptly instituted. Because metformin hydrochloride is dialyzable (with a clearance of up to 170 mL/min under good hemodynamic conditions), prompt hemodialysis is recommended to correct the acidosis and remove the accumulated metformin. Such management often results in prompt reversal of symptoms and recovery [see Contraindications; Warnings and Precautions].
Impaired Hepatic Function
Since impaired hepatic function has been associated with some cases of lactic acidosis, Janumet should generally be avoided in patients with clinical or laboratory evidence of hepatic disease.
Assessment of Renal Function
Metformin and sitagliptin are known to be substantially excreted by the kidney. The risk of metformin accumulation and lactic acidosis increases with the degree of impairment of renal function. Thus, patients with serum creatinine levels above the upper limit of normal for their age should not receive Janumet. In the elderly, Janumet should be carefully titrated to establish the minimum dose for adequate glycemic effect, because aging can be associated with reduced renal function. [See Warnings and Precautions and Use in Specific Populations.]
Before initiation of therapy with Janumet and at least annually thereafter, renal function should be assessed and verified as normal. In patients in whom development of renal dysfunction is anticipated, particularly in elderly patients, renal function should be assessed more frequently and Janumet discontinued if evidence of renal impairment is present.
Vitamin B12 Levels
In controlled clinical trials of metformin of 29 weeks duration, a decrease to subnormal levels of previously normal serum Vitamin B12 levels, without clinical manifestations, was observed in approximately 7% of patients. Such decrease, possibly due to interference with B12 absorption from the B12-intrinsic factor complex, is, however, very rarely associated with anemia and appears to be rapidly reversible with discontinuation of metformin or Vitamin B12 supplementation. Measurement of hematologic parameters on an annual basis is advised in patients on Janumet and any apparent abnormalities should be appropriately investigated and managed. [See Adverse Reactions.]
Certain individuals (those with inadequate Vitamin B12 or calcium intake or absorption) appear to be predisposed to developing subnormal Vitamin B12 levels. In these patients, routine serum Vitamin B12 measurements at two- to three-year intervals may be useful.
Alcohol Intake
Alcohol is known to potentiate the effect of metformin on lactate metabolism. Patients, therefore, should be warned against excessive alcohol intake, acute or chronic, while receiving Janumet.
Surgical Procedures
Use of Janumet should be temporarily suspended for any surgical procedure (except minor procedures not associated with restricted intake of food and fluids) and should not be restarted until the patient's oral intake has resumed and renal function has been evaluated as normal.
Change in Clinical Status of Patients with Previously Controlled Type 2 Diabetes
A patient with type 2 diabetes previously well controlled on Janumet who develops laboratory abnormalities or clinical illness (especially vague and poorly defined illness) should be evaluated promptly for evidence of ketoacidosis or lactic acidosis. Evaluation should include serum electrolytes and ketones, blood glucose and, if indicated, blood pH, lactate, pyruvate, and metformin levels. If acidosis of either form occurs, Janumet must be stopped immediately and other appropriate corrective measures initiated.
Use with Medications Known to Cause Hypoglycemia
Sitagliptin
As is typical with other antihyperglycemic agents used in combination with a sulfonylurea, when sitagliptin was used in combination with metformin and a sulfonylurea, a medication known to cause hypoglycemia, the incidence of hypoglycemia was increased over that of placebo in combination with metformin and a sulfonylurea [see Adverse Reactions]. Therefore, patients also receiving an insulin secretagogue (e.g., sulfonylurea, meglitinide) may require a lower dose of the insulin secretagogue to reduce the risk of hypoglycemia [see Dosage and Administration].
Metformin hydrochloride
Hypoglycemia does not occur in patients receiving metformin alone under usual circumstances of use, but could occur when caloric intake is deficient, when strenuous exercise is not compensated by caloric supplementation, or during concomitant use with other glucose-lowering agents (such as sulfonylureas and insulin) or ethanol. Elderly, debilitated, or malnourished patients, and those with adrenal or pituitary insufficiency or alcohol intoxication are particularly susceptible to hypoglycemic effects. Hypoglycemia may be difficult to recognize in the elderly, and in people who are taking β-adrenergic blocking drugs.
Concomitant Medications Affecting Renal Function or Metformin Disposition
Concomitant medication(s) that may affect renal function or result in significant hemodynamic change or may interfere with the disposition of metformin, such as cationic drugs that are eliminated by renal tubular secretion [see Drug Interactions], should be used with caution.
Radiologic Studies with Intravascular Iodinated Contrast Materials
Intravascular contrast studies with iodinated materials (for example, intravenous urogram, intravenous cholangiography, angiography, and computed tomography (CT) scans with intravascular contrast materials) can lead to acute alteration of renal function and have been associated with lactic acidosis in patients receiving metformin [see Contraindications]. Therefore, in patients in whom any such study is planned, Janumet should be temporarily discontinued at the time of or prior to the procedure, and withheld for 48 hours subsequent to the procedure and reinstituted only after renal function has been re-evaluated and found to be normal.
Hypoxic States
Cardiovascular collapse (shock) from whatever cause, acute congestive heart failure, acute myocardial infarction and other conditions characterized by hypoxemia have been associated with lactic acidosis and may also cause prerenal azotemia. When such events occur in patients on Janumet therapy, the drug should be promptly discontinued.
Loss of Control of Blood Glucose
When a patient stabilized on any diabetic regimen is exposed to stress such as fever, trauma, infection, or surgery, a temporary loss of glycemic control may occur. At such times, it may be necessary to withhold Janumet and temporarily administer insulin. Janumet may be reinstituted after the acute episode is resolved.
Hypersensitivity Reactions
There have been postmarketing reports of serious hypersensitivity reactions in patients treated with sitagliptin, one of the components of Janumet. These reactions include anaphylaxis, angioedema, and exfoliative skin conditions including Stevens-Johnson syndrome. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Onset of these reactions occurred within the first 3 months after initiation of treatment with sitagliptin, with some reports occurring after the first dose. If a hypersensitivity reaction is suspected, discontinue Janumet, assess for other potential causes for the event, and institute alternative treatment for diabetes. [See Adverse Reactions.]
Macrovascular Outcomes
There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with Janumet or any other anti-diabetic drug.
top
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Sitagliptin and Metformin Co-administration in Patients with Type 2 Diabetes Inadequately Controlled on Diet and Exercise
Table 1 summarizes the most common (≥5% of patients) adverse reactions reported (regardless of investigator assessment of causality) in a 24-week placebo-controlled factorial study in which sitagliptin and metformin were co-administered to patients with type 2 diabetes inadequately controlled on diet and exercise.
Table 1: Sitagliptin and Metformin Co-administered to Patients with Type 2 Diabetes Inadequately Controlled on Diet and Exercise: Adverse Reactions Reported (Regardless of Investigator Assessment of Causality) in ≥5% of Patients Receiving Combination Therapy (and Greater than in Patients Receiving Placebo)*
| Number of Patients (%) |
| | Placebo | Sitagliptin 100 mg QD | Metformin 500 mg/ Metformin 1000 mg bid†| Sitagliptin 50 mg bid + Metformin 500 mg/ Metformin 1000 mg bid†|
| | N = 176 | N = 179 | N = 364†| N = 372†|
|
| Diarrhea | 7 (4.0) | 5 (2.8) | 28 (7.7) | 28 (7.5) |
| Upper Respiratory Tract Infection | 9 (5.1) | 8 (4.5) | 19 (5.2) | 23 (6.2) |
| Headache | 5 (2.8) | 2 (1.1) | 14 (3.8) | 22 (5.9) |
Sitagliptin Add-on Therapy in Patients with Type 2 Diabetes Inadequately Controlled on Metformin Alone
In a 24-week placebo-controlled trial of sitagliptin 100 mg administered once daily added to a twice daily metformin regimen, there were no adverse reactions reported regardless of investigator assessment of causality in ≥5% of patients and more commonly than in patients given placebo. Discontinuation of therapy due to clinical adverse reactions was similar to the placebo treatment group (sitagliptin and metformin, 1.9%; placebo and metformin, 2.5%).
Hypoglycemia
Adverse reactions of hypoglycemia were based on all reports of hypoglycemia; a concurrent glucose measurement was not required. The overall incidence of pre-specified adverse reactions of hypoglycemia in patients with type 2 diabetes inadequately controlled on diet and exercise was 0.6% in patients given placebo, 0.6% in patients given sitagliptin alone, 0.8% in patients given metformin alone, and 1.6% in patients given sitagliptin in combination with metformin. In patients with type 2 diabetes inadequately controlled on metformin alone, the overall incidence of adverse reactions of hypoglycemia was 1.3% in patients given add-on sitagliptin and 2.1% in patients given add-on placebo.
Gastrointestinal Adverse Reactions
The incidences of pre-selected gastrointestinal adverse experiences in patients treated with sitagliptin and metformin were similar to those reported for patients treated with metformin alone. See Table 2.
Table 2: Pre-selected Gastrointestinal Adverse Reactions (Regardless of Investigator Assessment of Causality) Reported in Patients with Type 2 Diabetes Receiving Sitagliptin and Metformin.
| Number of Patients (%) |
| | Study of Sitagliptin and Metformin in Patients Inadequately Controlled
on Diet and Exercise | Study of Sitagliptin Add-on in Patients Inadequately Controlled on Metformin Alone |
| | Placebo | Sitagliptin 100 mg QD | Metformin 500 mg/ Metformin 1000 mg bid* | Sitagliptin 50 mg bid + Metformin 500 mg/ Metformin 1000 mg bid* | Placebo and Metformin ≥1500 mg daily | Sitagliptin 100 mg QD and Metformin ≥1500 mg daily |
| | N = 176 | N = 179 | N = 364 | N = 372 | N = 237 | N = 464 |
|
| Diarrhea | 7 (4.0) | 5 (2.8) | 28 (7.7) | 28 (7.5) | 6 (2.5) | 11 (2.4) |
| Nausea | 2 (1.1) | 2 (1.1) | 20 (5.5) | 18 (4.8) | 2 (0.8) | 6 (1.3) |
| Vomiting | 1 (0.6) | 0 (0.0) | 2 (0.5) | 8 (2.2) | 2 (0.8) | 5 (1.1) |
| Abdominal Pain†| 4 (2.3) | 6 (3.4) | 14 (3.8) | 11 (3.0) | 9 (3.8) | 10 (2.2) |
Sitagliptin in Combination with Metformin and Glimepiride
In a 24-week placebo-controlled study of sitagliptin 100 mg as add-on therapy in patients with type 2 diabetes inadequately controlled on metformin and glimepiride (sitagliptin, N=116; placebo, N=113), the adverse reactions reported regardless of investigator assessment of causality in ≥5% of patients treated with sitagliptin and more commonly than in patients treated with placebo were: hypoglycemia (sitagliptin, 16.4%; placebo, 0.9%) and headache (6.9%, 2.7%).
No clinically meaningful changes in vital signs or in ECG (including in QTc interval) were observed with the combination of sitagliptin and metformin.
The most common adverse experience in sitagliptin monotherapy reported regardless of investigator assessment of causality in ≥5% of patients and more commonly than in patients given placebo was nasopharyngitis.
The most common (>5%) established adverse reactions due to initiation of metformin therapy are diarrhea, nausea/vomiting, flatulence, abdominal discomfort, indigestion, asthenia, and headache.
Laboratory Tests
Sitagliptin
The incidence of laboratory adverse reactions was similar in patients treated with sitagliptin and metformin (7.6%) compared to patients treated with placebo and metformin (8.7%). In most but not all studies, a small increase in white blood cell count (approximately 200 cells/microL difference in WBC vs placebo; mean baseline WBC approximately 6600 cells/microL) was observed due to a small increase in neutrophils. This change in laboratory parameters is not considered to be clinically relevant.
Metformin hydrochloride
In controlled clinical trials of metformin of 29 weeks duration, a decrease to subnormal levels of previously normal serum Vitamin B12 levels, without clinical manifestations, was observed in approximately 7% of patients. Such decrease, possibly due to interference with B12 absorption from the B12-intrinsic factor complex, is, however, very rarely associated with anemia and appears to be rapidly reversible with discontinuation of metformin or Vitamin B12 supplementation. [See Warnings and Precautions.]
Postmarketing Experience
The following additional adverse reactions have been identified during postapproval use of Janumet or sitagliptin, one of the components of Janumet. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hypersensitivity reactions include anaphylaxis, angioedema, rash, urticaria, cutaneous vasculitis, and exfoliative skin conditions including Stevens-Johnson syndrome [see Warnings and Precautions]; upper respiratory tract infection; hepatic enzyme elevations; pancreatitis.
top
Drug Interactions
Cationic drugs
Cationic drugs (e.g., amiloride, digoxin, morphine, procainamide, quinidine, quinine, ranitidine, triamterene, trimethoprim, or vancomycin) that are eliminated by renal tubular secretion theoretically have the potential for interaction with metformin by competing for common renal tubular transport systems. Such interaction between metformin and oral cimetidine has been observed in normal healthy volunteers in both single- and multiple-dose metformin-cimetidine drug interaction studies, with a 60% increase in peak metformin plasma and whole blood concentrations and a 40% increase in plasma and whole blood metformin AUC. There was no change in elimination half-life in the single-dose study. Metformin had no effect on cimetidine pharmacokinetics. Although such interactions remain theoretical (except for cimetidine), careful patient monitoring and dose adjustment of Janumet and/or the interfering drug is recommended in patients who are taking cationic medications that are excreted via the proximal renal tubular secretory system.
Digoxin
There was a slight increase in the area under the curve (AUC, 11%) and mean peak drug concentration (Cmax, 18%) of digoxin with the co-administration of 100 mg sitagliptin for 10 days. These increases are not considered likely to be clinically meaningful. Digoxin, as a cationic drug, has the potential to compete with metformin for common renal tubular transport systems, thus affecting the serum concentrations of either digoxin, metformin or both. Patients receiving digoxin should be monitored appropriately. No dosage adjustment of digoxin or Janumet is recommended.
Glyburide
In a single-dose interaction study in type 2 diabetes patients, co-administration of metformin and glyburide did not result in any changes in either metformin pharmacokinetics or pharmacodynamics. Decreases in glyburide AUC and Cmax were observed, but were highly variable. The single-dose nature of this study and the lack of correlation between glyburide blood levels and pharmacodynamic effects make the clinical significance of this interaction uncertain.
Furosemide
A single-dose, metformin-furosemide drug interaction study in healthy subjects demonstrated that pharmacokinetic parameters of both compounds were affected by co-administration. Furosemide increased the metformin plasma and blood Cmax by 22% and blood AUC by 15%, without any significant change in metformin renal clearance. When administered with metformin, the Cmax and AUC of furosemide were 31% and 12% smaller, respectively, than when administered alone, and the terminal half-life was decreased by 32%, without any significant change in furosemide renal clearance. No information is available about the interaction of metformin and furosemide when co-administered chronically.
Nifedipine
A single-dose, metformin-nifedipine drug interaction study in normal healthy volunteers demonstrated that co-administration of nifedipine increased plasma metformin Cmax and AUC by 20% and 9%, respectively, and increased the amount excreted in the urine. Tmax and half-life were unaffected. Nifedipine appears to enhance the absorption of metformin. Metformin had minimal effects on nifedipine.
The Use of Metformin with Other Drugs
Certain drugs tend to produce hyperglycemia and may lead to loss of glycemic control. These drugs include the thiazides and other diuretics, corticosteroids, phenothiazines, thyroid products, estrogens, oral contraceptives, phenytoin, nicotinic acid, sympathomimetics, calcium channel blocking drugs, and isoniazid. When such drugs are administered to a patient receiving Janumet the patient should be closely observed to maintain adequate glycemic control.
In healthy volunteers, the pharmacokinetics of metformin and propranolol, and metformin and ibuprofen were not affected when co-administered in single-dose interaction studies.
Metformin is negligibly bound to plasma proteins and is, therefore, less likely to interact with highly protein-bound drugs such as salicylates, sulfonamides, chloramphenicol, and probenecid, as compared to the sulfonylureas, which are extensively bound to serum proteins.
top
Use in Specific Populations
Pregnancy
Pregnancy Category B:
Janumet
There are no adequate and well-controlled studies in pregnant women with Janumet or its individual components; therefore, the safety of Janumet in pregnant women is not known. Janumet should be used during pregnancy only if clearly needed.
Merck & Co., Inc. maintains a registry to monitor the pregnancy outcomes of women exposed to Janumet while pregnant. Health care providers are encouraged to report any prenatal exposure to Janumet by calling the Pregnancy Registry at (800) 986-8999.
No animal studies have been conducted with the combined products in Janumet to evaluate effects on reproduction. The following data are based on findings in studies performed with sitagliptin or metformin individually.
Sitagliptin
Reproduction studies have been performed in rats and rabbits. Doses of sitagliptin up to 125 mg/kg (approximately 12 times the human exposure at the maximum recommended human dose) did not impair fertility or harm the fetus. There are, however, no adequate and well-controlled studies with sitagliptin in pregnant women.
Sitagliptin administered to pregnant female rats and rabbits from gestation day 6 to 20 (organogenesis) was not teratogenic at oral doses up to 250 mg/kg (rats) and 125 mg/kg (rabbits), or approximately 30 and 20 times human exposure at the maximum recommended human dose (MRHD) of 100 mg/day based on AUC comparisons. Higher doses increased the incidence of rib malformations in offspring at 1000 mg/kg, or approximately 100 times human exposure at the MRHD.
Sitagliptin administered to female rats from gestation day 6 to lactation day 21 decreased body weight in male and female offspring at 1000 mg/kg. No functional or behavioral toxicity was observed in offspring of rats.
Placental transfer of sitagliptin administered to pregnant rats was approximately 45% at 2 hours and 80% at 24 hours postdose. Placental transfer of sitagliptin administered to pregnant rabbits was approximately 66% at 2 hours and 30% at 24 hours.
Metformin hydrochloride
Metformin was not teratogenic in rats and rabbits at doses up to 600 mg /kg/day. This represents an exposure of about 2 and 6 times the maximum recommended human daily dose of 2,000 mg based on body surface area comparisons for rats and rabbits, respectively. Determination of fetal concentrations demonstrated a partial placental barrier to metformin.
Nursing Mothers
No studies in lactating animals have been conducted with the combined components of Janumet. In studies performed with the individual components, both sitagliptin and metformin are secreted in the milk of lactating rats. It is not known whether sitagliptin is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Janumet is administered to a nursing woman.
Pediatric Use
Safety and effectiveness of Janumet in pediatric patients under 18 years have not been established.
Geriatric Use
Janumet
Because sitagliptin and metformin are substantially excreted by the kidney, and because aging can be associated with reduced renal function, Janumet should be used with caution as age increases. Care should be taken in dose selection and should be based on careful and regular monitoring of renal function. [See Warnings and Precautions; Clinical Pharmacology.]
Sitagliptin
Of the total number of subjects (N=3884) in Phase II and III clinical studies of sitagliptin, 725 patients were 65 years and over, while 61 patients were 75 years and over. No overall differences in safety or effectiveness were observed between subjects 65 years and over and younger subjects. While this and other reported clinical experience have not identified differences in responses between the elderly and younger patients, greater sensitivity of some older individuals cannot be ruled out.
Metformin hydrochloride
Controlled clinical studies of metformin did not include sufficient numbers of elderly patients to determine whether they respond differently from younger patients, although other reported clinical experience has not identified differences in responses between the elderly and young patients. Metformin should only be used in patients with normal renal function. The initial and maintenance dosing of metformin should be conservative in patients with advanced age, due to the potential for decreased renal function in this population. Any dose adjustment should be based on a careful assessment of renal function. [See Contraindications; Warnings and Precautions; and Clinical Pharmacology.]
top
Overdose
Sitagliptin
During controlled clinical trials in healthy subjects, single doses of up to 800 mg sitagliptin were administered. Maximal mean increases in QTc of 8.0 msec were observed in one study at a dose of 800 mg sitagliptin, a mean effect that is not considered clinically important [see Clinical Pharmacology]. There is no experience with doses above 800 mg in humans. In Phase I multiple-dose studies, there were no dose-related clinical adverse reactions observed with sitagliptin with doses of up to 400 mg per day for periods of up to 28 days.
In the event of an overdose, it is reasonable to employ the usual supportive measures, e.g., remove unabsorbed material from the gastrointestinal tract, employ clinical monitoring (including obtaining an electrocardiogram), and institute supportive therapy as indicated by the patient's clinical status.
Sitagliptin is modestly dialyzable. In clinical studies, approximately 13.5% of the dose was removed over a 3- to 4-hour hemodialysis session. Prolonged hemodialysis may be considered if clinically appropriate. It is not known if sitagliptin is dialyzable by peritoneal dialysis.
Metformin hydrochloride
Overdose of metformin hydrochloride has occurred, including ingestion of amounts greater than 50 grams. Hypoglycemia was reported in approximately 10% of cases, but no causal association with metformin hydrochloride has been established. Lactic acidosis has been reported in approximately 32% of metformin overdose cases [see Warnings and Precautions]. Metformin is dialyzable with a clearance of up to 170 mL/min under good hemodynamic conditions. Therefore, hemodialysis may be useful for removal of accumulated drug from patients in whom metformin overdosage is suspected.
top
Description
Janumet (sitagliptin/metformin HCl) tablets contain two oral antihyperglycemic drugs used in the management of type 2 diabetes: sitagliptin and metformin hydrochloride.
Sitagliptin
Sitagliptin is an orally-active inhibitor of the dipeptidyl peptidase-4 (DPP-4) enzyme. Sitagliptin is present in Janumet tablets in the form of sitagliptin phosphate monohydrate. Sitagliptin phosphate monohydrate is described chemically as 7 - [(3R) - 3 - amino - 1 - oxo - 4 - (2,4,5 - trifluorophenyl)butyl] - 5,6,7,8 - tetrahydro - 3 - (trifluoromethyl) - 1,2,4 - triazolo[4,3 - a]pyrazine phosphate (1:1) monohydrate with an empirical formula of C16H15F6N5O-H3PO4-H2O and a molecular weight of 523.32. The structural formula is:
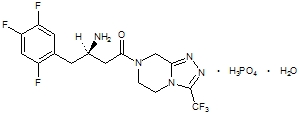
Sitagliptin phosphate monohydrate is a white to off-white, crystalline, non-hygroscopic powder. It is soluble in water and N,N-dimethyl formamide; slightly soluble in methanol; very slightly soluble in ethanol, acetone, and acetonitrile; and insoluble in isopropanol and isopropyl acetate.
Metformin hydrochloride
Metformin hydrochloride (N,N-dimethylimidodicarbonimidic diamide hydrochloride) is not chemically or pharmacologically related to any other classes of oral antihyperglycemic agents. Metformin hydrochloride is a white to off-white crystalline compound with a molecular formula of C4H11N5-HCl and a molecular weight of 165.63. Metformin hydrochloride is freely soluble in water and is practically insoluble in acetone, ether, and chloroform. The pKa of metformin is 12.4. The pH of a 1% aqueous solution of metformin hydrochloride is 6.68. The structural formula is as shown:
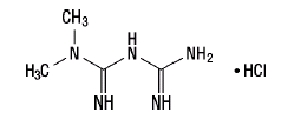
Janumet
Janumet is available for oral administration as tablets containing 64.25 mg sitagliptin phosphate monohydrate and metformin hydrochloride equivalent to: 50 mg sitagliptin as free base and 500 mg metformin hydrochloride (Janumet 50 mg/500 mg) or 1000 mg metformin hydrochloride (Janumet 50 mg/1000 mg). Each film-coated tablet of Janumet contains the following inactive ingredients: microcrystalline cellulose, polyvinylpyrrolidone, sodium lauryl sulfate, and sodium stearyl fumarate. In addition, the film coating contains the following inactive ingredients: polyvinyl alcohol, polyethylene glycol, talc, titanium dioxide, red iron oxide, and black iron oxide.
top
Clinical Pharmacology
Mechanism Of Action
Janumet
Janumet combines two antihyperglycemic agents with complementary mechanisms of action to improve glycemic control in patients with type 2 diabetes: sitagliptin, a dipeptidyl peptidase-4 (DPP-4) inhibitor, and metformin hydrochloride, a member of the biguanide class.
Sitagliptin
Sitagliptin is a DPP-4 inhibitor, which is believed to exert its actions in patients with type 2 diabetes by slowing the inactivation of incretin hormones. Concentrations of the active intact hormones are increased by sitagliptin, thereby increasing and prolonging the action of these hormones. Incretin hormones, including glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), are released by the intestine throughout the day, and levels are increased in response to a meal. These hormones are rapidly inactivated by the enzyme DPP-4. The incretins are part of an endogenous system involved in the physiologic regulation of glucose homeostasis. When blood glucose concentrations are normal or elevated, GLP-1 and GIP increase insulin synthesis and release from pancreatic beta cells by intracellular signaling pathways involving cyclic AMP. GLP-1 also lowers glucagon secretion from pancreatic alpha cells, leading to reduced hepatic glucose production. By increasing and prolonging active incretin levels, sitagliptin increases insulin release and decreases glucagon levels in the circulation in a glucose-dependent manner. Sitagliptin demonstrates selectivity for DPP-4 and does not inhibit DPP-8 or DPP-9 activity in vitro at concentrations approximating those from therapeutic doses.
Metformin hydrochloride
Metformin is an antihyperglycemic agent which improves glucose tolerance in patients with type 2 diabetes, lowering both basal and postprandial plasma glucose. Its pharmacologic mechanisms of action are different from other classes of oral antihyperglycemic agents. Metformin decreases hepatic glucose production, decreases intestinal absorption of glucose, and improves insulin sensitivity by increasing peripheral glucose uptake and utilization. Unlike sulfonylureas, metformin does not produce hypoglycemia in either patients with type 2 diabetes or normal subjects (except in special circumstances [see Warnings and Precautions]) and does not cause hyperinsulinemia. With metformin therapy, insulin secretion remains unchanged while fasting insulin levels and day-long plasma insulin response may actually decrease.
12.2 Pharmacodynamics
Sitagliptin
General
In patients with type 2 diabetes, administration of sitagliptin led to inhibition of DPP-4 enzyme activity for a 24-hour period. After an oral glucose load or a meal, this DPP-4 inhibition resulted in a 2- to 3-fold increase in circulating levels of active GLP-1 and GIP, decreased glucagon concentrations, and increased responsiveness of insulin release to glucose, resulting in higher C-peptide and insulin concentrations. The rise in insulin with the decrease in glucagon was associated with lower fasting glucose concentrations and reduced glucose excursion following an oral glucose load or a meal.
Sitagliptin and Metformin hydrochloride Co-administration
In a two-day study in healthy subjects, sitagliptin alone increased active GLP-1 concentrations, whereas metformin alone increased active and total GLP-1 concentrations to similar extents. Co-administration of sitagliptin and metformin had an additive effect on active GLP-1 concentrations. Sitagliptin, but not metformin, increased active GIP concentrations. It is unclear what these findings mean for changes in glycemic control in patients with type 2 diabetes.
In studies with healthy subjects, sitagliptin did not lower blood glucose or cause hypoglycemia.
Cardiac Electrophysiology
In a randomized, placebo-controlled crossover study, 79 healthy subjects were administered a single oral dose of sitagliptin 100 mg, sitagliptin 800 mg (8 times the recommended dose), and placebo. At the recommended dose of 100 mg, there was no effect on the QTc interval obtained at the peak plasma concentration, or at any other time during the study. Following the 800-mg dose, the maximum increase in the placebo-corrected mean change in QTc from baseline at 3 hours postdose was 8.0 msec. This increase is not considered to be clinically significant. At the 800-mg dose, peak sitagliptin plasma concentrations were approximately 11 times higher than the peak concentrations following a 100-mg dose.
In patients with type 2 diabetes administered sitagliptin 100 mg (N=81) or sitagliptin 200 mg (N=63) daily, there were no meaningful changes in QTc interval based on ECG data obtained at the time of expected peak plasma concentration.
Pharmacokinetics
Janumet
The results of a bioequivalence study in healthy subjects demonstrated that the Janumet (sitagliptin/metformin HCl) 50 mg/500 mg and 50 mg/1000 mg combination tablets are bioequivalent to co-administration of corresponding doses of sitagliptin (JANUVIA™2) and metformin hydrochloride as individual tablets.
Absorption
Sitagliptin
The absolute bioavailability of sitagliptin is approximately 87%. Co-administration of a high-fat meal with sitagliptin had no effect on the pharmacokinetics of sitagliptin.
Metformin hydrochloride
The absolute bioavailability of a metformin hydrochloride 500-mg tablet given under fasting conditions is approximately 50-60%. Studies using single oral doses of metformin hydrochloride tablets 500 mg to 1500 mg, and 850 mg to 2550 mg, indicate that there is a lack of dose proportionality with increasing doses, which is due to decreased absorption rather than an alteration in elimination. Food decreases the extent of and slightly delays the absorption of metformin, as shown by approximately a 40% lower mean peak plasma concentration (Cmax), a 25% lower area under the plasma concentration versus time curve (AUC), and a 35-minute prolongation of time to peak plasma concentration (Tmax) following administration of a single 850-mg tablet of metformin with food, compared to the same tablet strength administered fasting. The clinical relevance of these decreases is unknown.
Distribution
Sitagliptin
The mean volume of distribution at steady state following a single 100-mg intravenous dose of sitagliptin to healthy subjects is approximately 198 liters. The fraction of sitagliptin reversibly bound to plasma proteins is low (38%).
Metformin hydrochloride
The apparent volume of distribution (V/F) of metformin following single oral doses of metformin hydrochloride tablets 850 mg averaged 654 ± 358 L. Metformin is negligibly bound to plasma proteins, in contrast to sulfonylureas, which are more than 90% protein bound. Metformin partitions into erythrocytes, most likely as a function of time. At usual clinical doses and dosing schedules of metformin hydrochloride tablets, steady-state plasma concentrations of metformin are reached within 24-48 hours and are generally
Metabolism
Sitagliptin
Approximately 79% of sitagliptin is excreted unchanged in the urine with metabolism being a minor pathway of elimination.
Following a [14C]sitagliptin oral dose, approximately 16% of the radioactivity was excreted as metabolites of sitagliptin. Six metabolites were detected at trace levels and are not expected to contribute to the plasma DPP-4 inhibitory activity of sitagliptin. In vitro studies indicated that the primary enzyme responsible for the limited metabolism of sitagliptin was CYP3A4, with contribution from CYP2C8.
Metformin hydrochloride
Intravenous single-dose studies in normal subjects demonstrate that metformin is excreted unchanged in the urine and does not undergo hepatic metabolism (no metabolites have been identified in humans) nor biliary excretion.
Excretion
Sitagliptin
Following administration of an oral [14C]sitagliptin dose to healthy subjects, approximately 100% of the administered radioactivity was eliminated in feces (13%) or urine (87%) within one week of dosing. The apparent terminal t1/2 following a 100-mg oral dose of sitagliptin was approximately 12.4 hours and renal clearance was approximately 350 mL/min.
Elimination of sitagliptin occurs primarily via renal excretion and involves active tubular secretion. Sitagliptin is a substrate for human organic anion transporter-3 (hOAT-3), which may be involved in the renal elimination of sitagliptin. The clinical relevance of hOAT-3 in sitagliptin transport has not been established. Sitagliptin is also a substrate of p-glycoprotein, which may also be involved in mediating the renal elimination of sitagliptin. However, cyclosporine, a p-glycoprotein inhibitor, did not reduce the renal clearance of sitagliptin.
Metformin hydrochloride
Renal clearance is approximately 3.5 times greater than creatinine clearance, which indicates that tubular secretion is the major route of metformin elimination. Following oral administration, approximately 90% of the absorbed drug is eliminated via the renal route within the first 24 hours, with a plasma elimination half-life of approximately 6.2 hours. In blood, the elimination half-life is approximately 17.6 hours, suggesting that the erythrocyte mass may be a compartment of distribution.
Special Populations
Renal Insufficiency
Janumet
Janumet should not be used in patients with renal insufficiency [see Contraindications; Warnings and Precautions].
Sitagliptin
An approximately 2-fold increase in the plasma AUC of sitagliptin was observed in patients with moderate renal insufficiency, and an approximately 4-fold increase was observed in patients with severe renal insufficiency including patients with ESRD on hemodialysis, as compared to normal healthy control subjects.
Metformin hydrochloride
In patients with decreased renal function (based on measured creatinine clearance), the plasma and blood half-life of metformin is prolonged and the renal clearance is decreased in proportion to the decrease in creatinine clearance.
Hepatic Insufficiency
Sitagliptin
In patients with moderate hepatic insufficiency (Child-Pugh score 7 to 9), mean AUC and Cmax of sitagliptin increased approximately 21% and 13%, respectively, compared to healthy matched controls following administration of a single 100-mg dose of sitagliptin. These differences are not considered to be clinically meaningful.
There is no clinical experience in patients with severe hepatic insufficiency (Child-Pugh score >9).
Metformin hydrochloride
No pharmacokinetic studies of metformin have been conducted in patients with hepatic insufficiency.
Gender
Sitagliptin
Gender had no clinically meaningful effect on the pharmacokinetics of sitagliptin based on a composite analysis of Phase I pharmacokinetic data and on a population pharmacokinetic analysis of Phase I and Phase II data.
Metformin hydrochloride
Metformin pharmacokinetic parameters did not differ significantly between normal subjects and patients with type 2 diabetes when analyzed according to gender. Similarly, in controlled clinical studies in patients with type 2 diabetes, the antihyperglycemic effect of metformin was comparable in males and females.
Geriatric
Sitagliptin
When the effects of age on renal function are taken into account, age alone did not have a clinically meaningful impact on the pharmacokinetics of sitagliptin based on a population pharmacokinetic analysis. Elderly subjects (65 to 80 years) had approximately 19% higher plasma concentrations of sitagliptin compared to younger subjects.
Metformin hydrochloride
Limited data from controlled pharmacokinetic studies of metformin in healthy elderly subjects suggest that total plasma clearance of metformin is decreased, the half life is prolonged, and Cmax is increased, compared to healthy young subjects. From these data, it appears that the change in metformin pharmacokinetics with aging is primarily accounted for by a change in renal function (see GLUCOPHAGE3 prescribing information: CLINICAL PHARMACOLOGY, Special Populations, Geriatrics).
Janumet treatment should not be initiated in patients ≥80 years of age unless measurement of creatinine clearance demonstrates that renal function is not reduced [see Warnings and Precautions].
Pediatric
No studies with Janumet have been performed in pediatric patients.
Race
Sitagliptin
Race had no clinically meaningful effect on the pharmacokinetics of sitagliptin based on a composite analysis of available pharmacokinetic data, including subjects of white, Hispanic, black, Asian, and other racial groups.
Metformin hydrochloride
No studies of metformin pharmacokinetic parameters according to race have been performed. In controlled clinical studies of metformin in patients with type 2 diabetes, the antihyperglycemic effect was comparable in whites (n=249), blacks (n=51), and Hispanics (n=24).
Body Mass Index (BMI)
Sitagliptin
Body mass index had no clinically meaningful effect on the pharmacokinetics of sitagliptin based on a composite analysis of Phase I pharmacokinetic data and on a population pharmacokinetic analysis of Phase I and Phase II data.
Drug Interactions
Sitagliptin and Metformin hydrochloride
Co-administration of multiple doses of sitagliptin (50 mg) and metformin (1000 mg) given twice daily did not meaningfully alter the pharmacokinetics of either sitagliptin or metformin in patients with type 2 diabetes.
Pharmacokinetic drug interaction studies with Janumet have not been performed; however, such studies have been conducted with the individual components of Janumet (sitagliptin and metformin hydrochloride).
Sitagliptin
In Vitro Assessment of Drug Interactions
Sitagliptin is not an inhibitor of CYP isozymes CYP3A4, 2C8, 2C9, 2D6, 1A2, 2C19 or 2B6, and is not an inducer of CYP3A4. Sitagliptin is a p-glycoprotein substrate, but does not inhibit p-glycoprotein mediated transport of digoxin. Based on these results, sitagliptin is considered unlikely to cause interactions with other drugs that utilize these pathways.
Sitagliptin is not extensively bound to plasma proteins. Therefore, the propensity of sitagliptin to be involved in clinically meaningful drug-drug interactions mediated by plasma protein binding displacement is very low.
In Vivo Assessment of Drug Interactions
Effect of Sitagliptin on Other Drugs
In clinical studies, as described below, sitagliptin did not meaningfully alter the pharmacokinetics of metformin, glyburide, simvastatin, rosiglitazone, warfarin, or oral contraceptives, providing in vivo evidence of a low propensity for causing drug interactions with substrates of CYP3A4, CYP2C8, CYP2C9, and organic cationic transporter (OCT).
Digoxin: Sitagliptin had a minimal effect on the pharmacokinetics of digoxin. Following administration of 0.25 mg digoxin concomitantly with 100 mg of sitagliptin daily for 10 days, the plasma AUC of digoxin was increased by 11%, and the plasma Cmax by 18%.
Sulfonylureas: Single-dose pharmacokinetics of glyburide, a CYP2C9 substrate, was not meaningfully altered in subjects receiving multiple doses of sitagliptin. Clinically meaningful interactions would not be expected with other sulfonylureas (e.g., glipizide, tolbutamide, and glimepiride) which, like glyburide, are primarily eliminated by CYP2C9 [see Warnings and Precautions].
Simvastatin: Single-dose pharmacokinetics of simvastatin, a CYP3A4 substrate, was not meaningfully altered in subjects receiving multiple daily doses of sitagliptin. Therefore, sitagliptin is not an inhibitor of CYP3A4-mediated metabolism.
Thiazolidinediones: Single-dose pharmacokinetics of rosiglitazone was not meaningfully altered in subjects receiving multiple daily doses of sitagliptin, indicating that sitagliptin is not an inhibitor of CYP2C8-mediated metabolism.
Warfarin: Multiple daily doses of sitagliptin did not meaningfully alter the pharmacokinetics, as assessed by measurement of S(-) or R(+) warfarin enantiomers, or pharmacodynamics (as assessed by measurement of prothrombin INR) of a single dose of warfarin. Because S(-) warfarin is primarily metabolized by CYP2C9, these data also support the conclusion that sitagliptin is not a CYP2C9 inhibitor.
Oral Contraceptives: Co-administration with sitagliptin did not meaningfully alter the steady-state pharmacokinetics of norethindrone or ethinyl estradiol.
Effect of Other Drugs on Sitagliptin
Clinical data described below suggest that sitagliptin is not susceptible to clinically meaningful interactions by co-administered medications.
Cyclosporine: A study was conducted to assess the effect of cyclosporine, a potent inhibitor of p-glycoprotein, on the pharmacokinetics of sitagliptin. Co-administration of a single 100-mg oral dose of sitagliptin and a single 600-mg oral dose of cyclosporine increased the AUC and Cmax of sitagliptin by approximately 29% and 68%, respectively. These modest changes in sitagliptin pharmacokinetics were not considered to be clinically meaningful. The renal clearance of sitagliptin was also not meaningfully altered. Therefore, meaningful interactions would not be expected with other p-glycoprotein inhibitors.
Metformin hydrochloride
[See Drug Interactions]
top
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment Of Fertility
Janumet
No animal studies have been conducted with the combined products in Janumet to evaluate carcinogenesis, mutagenesis or impairment of fertility. The following data are based on the findings in studies with sitagliptin and metformin individually.
Sitagliptin
A two-year carcinogenicity study was conducted in male and female rats given oral doses of sitagliptin of 50, 150, and 500 mg/kg/day. There was an increased incidence of combined liver adenoma/carcinoma in males and females and of liver carcinoma in females at 500 mg/kg. This dose results in exposures approximately 60 times the human exposure at the maximum recommended daily adult human dose (MRHD) of 100 mg/day based on AUC comparisons. Liver tumors were not observed at 150 mg/kg, approximately 20 times the human exposure at the MRHD. A two-year carcinogenicity study was conducted in male and female mice given oral doses of sitagliptin of 50, 125, 250, and 500 mg/kg/day. There was no increase in the incidence of tumors in any organ up to 500 mg/kg, approximately 70 times human exposure at the MRHD. Sitagliptin was not mutagenic or clastogenic with or without metabolic activation in the Ames bacterial mutagenicity assay, a Chinese hamster ovary (CHO) chromosome aberration assay, an in vitro cytogenetics assay in CHO, an in vitro rat hepatocyte DNA alkaline elution assay, and an in vivo micronucleus assay.
In rat fertility studies with oral gavage doses of 125, 250, and 1000 mg/kg, males were treated for 4 weeks prior to mating, during mating, up to scheduled termination (approximately 8 weeks total), and females were treated 2 weeks prior to mating through gestation day 7. No adverse effect on fertility was observed at 125 mg/kg (approximately 12 times human exposure at the MRHD of 100 mg/day based on AUC comparisons). At higher doses, nondose-related increased resorptions in females were observed (approximately 25 and 100 times human exposure at the MRHD based on AUC comparison).
Metformin hydrochloride
Long-term carcinogenicity studies have been performed in rats (dosing duration of 104 weeks) and mice (dosing duration of 91 weeks) at doses up to and including 900 mg/kg/day and 1500 mg/kg/day, respectively. These doses are both approximately four times the maximum recommended human daily dose of 2000 mg based on body surface area comparisons. No evidence of carcinogenicity with metformin was found in either male or female mice. Similarly, there was no tumorigenic potential observed with metformin in male rats. There was, however, an increased incidence of benign stromal uterine polyps in female rats treated with 900 mg/kg/day.
There was no evidence of a mutagenic potential of metformin in the following in vitro tests: Ames test (S. typhimurium), gene mutation test (mouse lymphoma cells), or chromosomal aberrations test (human lymphocytes). Results in the in vivo mouse micronucleus test were also negative. Fertility of male or female rats was unaffected by metformin when administered at doses as high as 600 mg/kg/day, which is approximately three times the maximum recommended human daily dose based on body surface area comparisons.
top
Clinical Studies
The co-administration of sitagliptin and metformin has been studied in patients with type 2 diabetes inadequately controlled on diet and exercise and in combination with glimepiride.
There have been no clinical efficacy studies conducted with Janumet; however, bioequivalence of Janumet with co-administered sitagliptin and metformin hydrochloride tablets was demonstrated.
Sitagliptin and Metformin Co-administration in Patients with Type 2 Diabetes Inadequately Controlled on Diet and Exercise
A total of 1091 patients with type 2 diabetes and inadequate glycemic control on diet and exercise participated in a 24-week, randomized, double-blind, placebo-controlled factorial study designed to assess the efficacy of sitagliptin and metformin co-administration. Patients on an antihyperglycemic agent (N=541) underwent a diet, exercise, and drug washout period of up to 12 weeks duration. After the washout period, patients with inadequate glycemic control (A1C 7.5% to 11%) were randomized after completing a 2-week single-blind placebo run-in period. Patients not on antihyperglycemic agents at study entry (N=550) with inadequate glycemic control (A1C 7.5% to 11%) immediately entered the 2-week single-blind placebo run-in period and then were randomized. Approximately equal numbers of patients were randomized to receive placebo, 100 mg of sitagliptin once daily, 500 mg or 1000 mg of metformin twice daily, or 50 mg of sitagliptin twice daily in combination with 500 mg or 1000 mg of metformin twice daily. Patients who failed to meet specific glycemic goals during the study were treated with glyburide (glibenclamide) rescue.
Sitagliptin and metformin co-administration provided significant improvements in A1C, FPG, and 2-hour PPG compared to placebo, to metformin alone, and to sitagliptin alone (Table 3, Figure 1). Mean reductions from baseline in A1C were generally greater for patients with higher baseline A1C values. For patients not on an antihyperglycemic agent at study entry, mean reductions from baseline in A1C were: sitagliptin 100 mg once daily, -1.1%; metformin 500 mg bid, -1.1%; metformin 1000 mg bid, -1.2%; sitagliptin 50 mg bid with metformin 500 mg bid, -1.6%; sitagliptin 50 mg bid with metformin 1000 mg bid, -1.9%; and for patients receiving placebo, -0.2%. Lipid effects were generally neutral. The decrease in body weight in the groups given sitagliptin in combination with metformin was similar to that in the groups given metformin alone or placebo.
Table 3: Glycemic Parameters at Final Visit (24-Week Study) for Sitagliptin and Metformin, Alone and in Combination in Patients with Type 2 Diabetes Inadequately Controlled on Diet and Exercise*
| Placebo | Sitagliptin 100 mg QD | Metformin
500 mg bid | Metformin
1000 mg bid | Sitagliptin
50 mg bid +
Metformin
500 mg bid | Sitagliptin
50 mg bid +
Metformin
1000 mg bid |
|
| A1C (%) | N = 165 | N = 175 | N = 178 | N = 177 | N = 183 | N = 178 |
| Baseline (mean) | 8.7 | 8.9 | 8.9 | 8.7 | 8.8 | 8.8 |
| Change from baseline (adjusted mean†) | 0.2 | -0.7 | -0.8 | -1.1 | -1.4 | -1.9 |
Difference from placebo (adjusted mean†)
(95% CI) | | -0.8c
(-1.1, -0.6) | -1.0c
(-1.2, -0.8) | -1.3c
(-1.5, -1.1) | -1.6c
(-1.8, -1.3) | -2.1c
(-2.3, -1.8) |
| Patients (%) achieving A1C <7% | 15 (9%) | 35 (20%) | 41 (23%) | 68 (38%) | 79 (43%) | 118 (66%) |
| % Patients receiving rescue medication | 32 | 21 | 17 | 12 | 8 | 2 |
| FPG (mg/dL) | N = 169 | N = 178 | N = 179 | N = 179 | N = 183 | N = 180 |
| Baseline (mean) | 196 | 201 | 205 | 197 | 204 | 197 |
| Change from baseline (adjusted mean†) | 6 | -17 | -27 | -29 | -47 | -64 |
Difference from placebo (adjusted mean†)
(95% CI) | | -23c
(-33, -14) | -33c
(-43, -24) | -35c
(-45, -26) | -53c
(-62, -43) | -70c
(-79, -60) |
| 2-hour PPG (mg/dL) | N = 129 | N = 136 | N = 141 | N = 138 | N = 147 | N = 152 |
| Baseline (mean) | 277 | 285 | 293 | 283 | 292 | 287 |
| Change from baseline (adjusted mean†) | 0 | -52 | -53 | -78 | -93 | -117 |
Difference from placebo (adjusted mean†)
(95% CI) | | -52c
(-67, -37) | -54c
(-69, -39) | -78c
(-93, -63) | -93c
(-107, -78) | -117c
(-131, -102) |
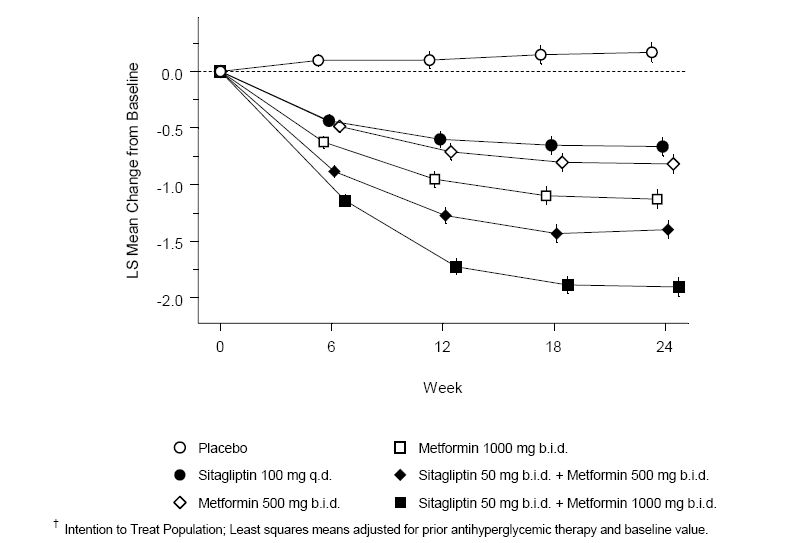
Figure 1: Mean Change from Baseline for A1C (%) over 24 Weeks with Sitagliptin and Metformin, Alone and in Combination in Patients with Type 2 Diabetes Inadequately Controlled with Diet and Exerciseâ€
In addition, this study included patients (N=117) with more severe hyperglycemia (A1C >11% or blood glucose >280 mg/dL) who were treated with twice daily open-label sitagliptin 50 mg and metformin 1000 mg. In this group of patients, the mean baseline A1C value was 11.2%, mean FPG was 314 mg/dL, and mean 2-hour PPG was 441 mg/dL. After 24 weeks, mean decreases from baseline of -2.9% for A1C, -127 mg/dL for FPG, and -208 mg/dL for 2-hour PPG were observed.
Initial combination therapy or maintenance of combination therapy should be individualized and are left to the discretion of the health care provider.
Sitagliptin Add-on Therapy in Patients with Type 2 Diabetes Inadequately Controlled on Metformin Alone
A total of 701 patients with type 2 diabetes participated in a 24-week, randomized, double-blind, placebo-controlled study designed to assess the efficacy of sitagliptin in combination with metformin. Patients already on metformin (N=431) at a dose of at least 1500 mg per day were randomized after completing a 2-week, single-blind placebo run-in period. Patients on metformin and another antihyperglycemic agent (N=229) and patients not on any antihyperglycemic agents (off therapy for at least 8 weeks, N=41) were randomized after a run-in period of approximately 10 weeks on metformin (at a dose of at least 1500 mg per day) in monotherapy. Patients were randomized to the addition of either 100 mg of sitagliptin or placebo, administered once daily. Patients who failed to meet specific glycemic goals during the studies were treated with pioglitazone rescue.
In combination with metformin, sitagliptin provided significant improvements in A1C, FPG, and 2-hour PPG compared to placebo with metformin (Table 4). Rescue glycemic therapy was used in 5% of patients treated with sitagliptin 100 mg and 14% of patients treated with placebo. A similar decrease in body weight was observed for both treatment groups.
Table 4: Glycemic Parameters at Final Visit (24-Week Study) of Sitagliptin in Add-on Combination Therapy with Metformin*
| | Sitagliptin 100 mg QD
+ Metformin | Placebo
+ Metformin |
|
| A1C (%) | N = 453 | N = 224 |
| Baseline (mean) | 8.0 | 8.0 |
| Change from baseline (adjusted mean†) | -0.7 | -0.0 |
Difference from placebo + metformin (adjusted mean†)
(95% CI) | -0.7c
(-0.8, -0.5) | |
| Patients (%) achieving A1C <7% | 213 (47%) | 41 (18%) |
| FPG (mg/dL) | N = 454 | N = 226 |
| Baseline (mean) | 170 | 174 |
| Change from baseline (adjusted mean†) | -17 | 9 |
Difference from placebo + metformin (adjusted mean†)
(95% CI) | -25c
(-31, -20) | |
| 2-hour PPG (mg/dL) | N = 387 | N = 182 |
| Baseline (mean) | 275 | 272 |
| Change from baseline (adjusted mean†) | -62 | -11 |
Difference from placebo + metformin (adjusted mean†)
(95% CI) | -51c
(-61, -41) | |
Sitagliptin Add-on Therapy in Patients with Type 2 Diabetes Inadequately Controlled on the Combination of Metformin and Glimepiride
A total of 441 patients with type 2 diabetes participated in a 24-week, randomized, double-blind, placebo-controlled study designed to assess the efficacy of sitagliptin in combination with glimepiride, with or without metformin. Patients entered a run-in treatment period on glimepiride (≥4 mg per day) alone or glimepiride in combination with metformin (≥1500 mg per day). After a dose-titration and dose-stable run-in period of up to 16 weeks and a 2-week placebo run-in period, patients with inadequate glycemic control (A1C 7.5% to 10.5%) were randomized to the addition of either 100 mg of sitagliptin or placebo, administered once daily. Patients who failed to meet specific glycemic goals during the studies were treated with pioglitazone rescue.
Patients receiving sitagliptin with metformin and glimepiride had significant improvements in A1C and FPG compared to patients receiving placebo with metformin and glimepiride (Table 5), with mean reductions from baseline relative to placebo in A1C of -0.9% and in FPG of -21 mg/dL. Rescue therapy was used in 8% of patients treated with sitagliptin 100 mg and 29% of patients treated with add-on placebo. The patients treated with add-on sitagliptin had a mean increase in body weight of 1.1 kg vs. add-on placebo (+0.4 kg vs. -0.7 kg). In addition, add-on sitagliptin resulted in an increased rate of hypoglycemia compared to add-on placebo. [See Warnings and Precautions; Adverse Reactions.]
Table 5: Glycemic Parameters at Final Visit (24-Week Study) for Sitagliptin in Combination with Metformin and Glimepiride*
Sitagliptin 100 mg
+ Metformin
and Glimepiride | Placebo
+ Metformin
and Glimepiride |
|
| A1C (%) | N = 115 | N = 105 |
| Baseline (mean) | 8.3 | 8.3 |
| Change from baseline (adjusted mean†) | -0.6 | 0.3 |
| Difference from placebo (adjusted mean†) (95% CI) | -0.9c
(-1.1, -0.7) | |
| Patients (%) achieving A1C <7% | 26 (23%) | 1 (1%) |
| FPG (mg/dL) | N = 115 | N = 109 |
| Baseline (mean) | 179 | 179 |
| Change from baseline (adjusted mean†) | -8 | 13 |
| Difference from placebo (adjusted mean†) (95% CI) | -21c
(-32, -10) | |
Sitagliptin Add-on Therapy vs. Glipizide Add-on Therapy in Patients with Type 2 Diabetes Inadequately Controlled on Metformin
The efficacy of sitagliptin was evaluated in a 52-week, double-blind, glipizide-controlled noninferiority trial in patients with type 2 diabetes. Patients not on treatment or on other antihyperglycemic agents entered a run-in treatment period of up to 12 weeks duration with metformin monotherapy (dose of ≥1500 mg per day) which included washout of medications other than metformin, if applicable. After the run-in period, those with inadequate glycemic control (A1C 6.5% to 10%) were randomized 1:1 to the addition of sitagliptin 100 mg once daily or glipizide for 52 weeks. Patients receiving glipizide were given an initial dosage of 5 mg/day and then electively titrated over the next 18 weeks to a maximum dosage of 20 mg/day as needed to optimize glycemic control. Thereafter, the glipizide dose was to be kept constant, except for down-titration to prevent hypoglycemia. The mean dose of glipizide after the titration period was 10 mg.
After 52 weeks, sitagliptin and glipizide had similar mean reductions from baseline in A1C in the intent-to-treat analysis (Table 6). These results were consistent with the per protocol analysis (Figure 2). A conclusion in favor of the non-inferiority of sitagliptin to glipizide may be limited to patients with baseline A1C comparable to those included in the study (over 70% of patients had baseline A1C <8% and over 90% had A1C <9%).
Table 6: Glycemic Parameters in a 52-Week Study Comparing Sitagliptin to Glipizide as Add-On Therapy in Patients Inadequately Controlled on Metformin (Intent-to-Treat Population)*
| | Sitagliptin 100 mg
+ Metformin | Glipizide
+ Metformin |
|
| A1C (%) | N = 576 | N = 559 |
| Baseline (mean) | 7.7 | 7.6 |
| Change from baseline (adjusted mean†) | -0.5 | -0.6 |
| FPG (mg/dL) | N = 583 | N = 568 |
| Baseline (mean) | 166 | 164 |
| Change from baseline (adjusted mean†) | -8 | -8 |
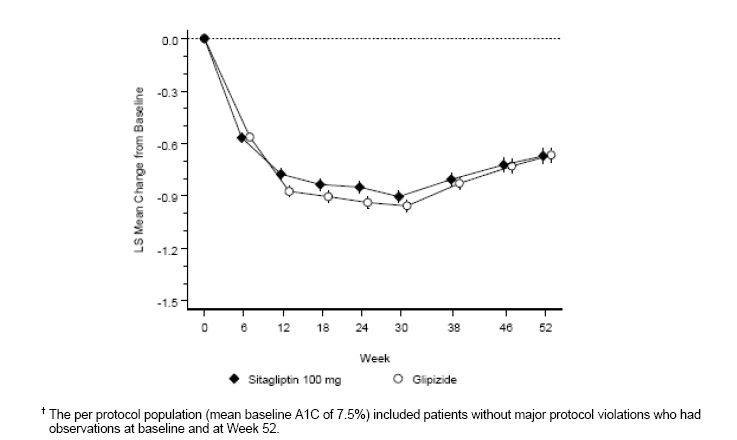
Figure 2: Mean Change from Baseline for A1C (%) Over 52 Weeks in a Study Comparing Sitagliptin to Glipizide as Add-On Therapy in Patients Inadequately Controlled on Metformin (Per Protocol Population)â€
The incidence of hypoglycemia in the sitagliptin group (4.9%) was significantly (p<0.001) lower than that in the glipizide group (32.0%). Patients treated with sitagliptin exhibited a significant mean decrease from baseline in body weight compared to a significant weight gain in patients administered glipizide (-1.5 kg vs. +1.1 kg).
top
How Supplied
No. 6747 - Tablets Janumet, 50 mg/500 mg, are light pink, capsule-shaped, film-coated tablets with "575" debossed on one side. They are supplied as follows:
NDC 0006-0575-61 unit-of-use bottles of 60
NDC 0006-0575-62 unit-of-use bottles of 180
NDC 0006-0575-52 unit dose blister packages of 50
NDC 0006-0575-82 bulk bottles of 1000.
No. 6749 — Tablets Janumet, 50 mg/1000 mg, are red, capsule-shaped, film-coated tablets with "577" debossed on one side. They are supplied as follows:
NDC 0006-0577-61 unit-of-use bottles of 60
NDC 0006-0577-62 unit-of-use bottles of 180
NDC 0006-0577-52 unit dose blister packages of 50
NDC 0006-0577-82 bulk bottles of 1000.
Store at 20-25°C (68-77°F), excursions permitted to 15-30°C (59-86°F).
top
Patient Counseling Information
Instructions
Patients should be informed of the potential risks and benefits of Janumet and of alternative modes of therapy. They should also be informed about the importance of adherence to dietary instructions, regular physical activity, periodic blood glucose monitoring and A1C testing, recognition and management of hypoglycemia and hyperglycemia, and assessment for diabetes complications. During periods of stress such as fever, trauma, infection, or surgery, medication requirements may change and patients should be advised to seek medical advice promptly.
The risks of lactic acidosis due to the metformin component, its symptoms, and conditions that predispose to its development, as noted in Warnings and Precautions, should be explained to patients. Patients should be advised to discontinue Janumet immediately and to promptly notify their health practitioner if unexplained hyperventilation, myalgia, malaise, unusual somnolence, dizziness, slow or irregular heart beat, sensation of feeling cold (especially in the extremities) or other nonspecific symptoms occur. Gastrointestinal symptoms are common during initiation of metformin treatment and may occur during initiation of Janumet therapy; however, patients should consult their physician if they develop unexplained symptoms. Although gastrointestinal symptoms that occur after stabilization are unlikely to be drug related, such an occurrence of symptoms should be evaluated to determine if it may be due to lactic acidosis or other serious disease.
Patients should be counseled against excessive alcohol intake, either acute or chronic, while receiving Janumet.
Patients should be informed about the importance of regular testing of renal function and hematological parameters when receiving treatment with Janumet.
Patients should be informed that allergic reactions have been reported during postmarketing use of sitagliptin, one of the components of Janumet. If symptoms of allergic reactions (including rash, hives, and swelling of the face, lips, tongue, and throat that may cause difficulty in breathing or swallowing) occur, patients must stop taking Janumet and seek medical advice promptly.
Physicians should instruct their patients to read the Patient Package Insert before starting Janumet therapy and to reread each time the prescription is renewed. Patients should be instructed to inform their doctor or pharmacist if they develop any unusual symptom, or if any known symptom persists or worsens.
Laboratory Tests
Response to all diabetic therapies should be monitored by periodic measurements of blood glucose and A1C levels, with a goal of decreasing these levels towards the normal range. A1C is especially useful for evaluating long-term glycemic control.
Initial and periodic monitoring of hematologic parameters (e.g., hemoglobin/hematocrit and red blood cell indices) and renal function (serum creatinine) should be performed, at least on an annual basis. While megaloblastic anemia has rarely been seen with metformin therapy, if this is suspected, Vitamin B12 deficiency should be excluded.
Distributed by:
MERCK & CO., INC., Whitehouse Station, NJ 08889, USA
9794108
US Patent No.: 6,699,871
1Registered trademark of MERCK & CO., Inc., Whitehouse Station, New Jersey 08889 USA
2Trademark of MERCK & CO., Inc., Whitehouse Station, New Jersey 08889 USA
3GLUCOPHAGE® is a registered trademark of Merck Sante S.A.S, an associate of Merck KGaA of Darmstadt, Germany.
Licensed to Bristol-Myers Squibb Company.
COPYRIGHT © 2007, 2008 MERCK & CO., Inc.
All rights reserved
FDA-Approved Patient Labeling
Patient Information
Janumet® (JAN-you-met)
(sitagliptin/metformin HCl)
Tablets
Read the Patient Information that comes with Janumet1 before you start taking it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about your medical condition or treatment.
What is the most important information I should know about Janumet?
Metformin hydrochloride, one of the ingredients in Janumet, can cause a rare but serious side effect called lactic acidosis (a build-up of lactic acid in the blood) that can cause death. Lactic acidosis is a medical emergency and must be treated in a hospital.
Stop taking Janumet and call your doctor right away if you get any of the following symptoms of lactic acidosis:
- You feel very weak and tired.
- You have unusual (not normal) muscle pain.
- You have trouble breathing.
- You have unexplained stomach or intestinal problems with nausea and vomiting, or diarrhea.
- You feel cold, especially in your arms and legs.
- You feel dizzy or lightheaded.
- You have a slow or irregular heart beat.
You have a higher chance of getting lactic acidosis if you:
- have kidney problems.
- have liver problems.
- have congestive heart failure that requires treatment with medicines.
- drink a lot of alcohol (very often or short-term "binge" drinking).
- get dehydrated (lose a large amount of body fluids). This can happen if you are sick with a fever, vomiting, or diarrhea. Dehydration can also happen when you sweat a lot with activity or exercise and don't drink enough fluids.
- have certain x-ray tests with injectable dyes or contrast agents.
- have surgery.
- have a heart attack, severe infection, or stroke.
- are 80 years of age or older and have not had your kidney function tested.
What is Janumet?
Janumet tablets contain two prescription medicines, sitagliptin (JANUVIA™2) and metformin. Janumet can be used along with diet and exercise to lower blood sugar in adult patients with type 2 diabetes. Your doctor will determine if Janumet is right for you and will determine the best way to start and continue to treat your diabetes.
Janumet:
- helps to improve the levels of insulin after a meal.
- helps the body respond better to the insulin it makes naturally.
- decreases the amount of sugar made by the body.
- is unlikely to cause low blood sugar (hypoglycemia) when it is taken by itself to treat high blood sugar.
Janumet has not been studied in children under 18 years of age.
Janumet has not been studied with insulin, a medicine known to cause low blood sugar.
Who should not take Janumet?
Do not take Janumet if you:
- have type 1 diabetes.
- have certain kidney problems.
- have conditions called metabolic acidosis or diabetic ketoacidosis (increased ketones in the blood or urine).
- have had an allergic reaction to Janumet or sitagliptin (JANUVIA), one of the components of Janumet.
- are going to receive an injection of dye or contrast agents for an x-ray procedure, Janumet will need to be stopped for a short time. Talk to your doctor about when to stop Janumet and when to start again. See "What is the most important information I should know about Janumet?"
What should I tell my doctor before and during treatment with Janumet?
Janumet may not be right for you. Tell your doctor about all of your medical conditions, including if you:
- have kidney problems.
- have liver problems.
- have had an allergic reaction to Janumet or sitagliptin (JANUVIA), one of the components of Janumet.
- have heart problems, including congestive heart failure.
- are older than 80 years. Patients over 80 years should not take Janumet unless their kidney function is checked and it is normal.
- drink alcohol a lot (all the time or short-term "binge" drinking).
- are pregnant or plan to become pregnant. It is not known if Janumet will harm your unborn baby. If you are pregnant, talk with your doctor about the best way to control your blood sugar while you are pregnant. If you use Janumet during pregnancy, talk with your doctor about how you can be on the Janumet registry. The toll-free telephone number for the pregnancy registry is 1-800-986-8999.
- are breast-feeding or plan to breast-feed. It is not known if Janumet will pass into your breast milk. Talk with your doctor about the best way to feed your baby if you are taking Janumet.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Janumet may affect how well other drugs work and some drugs can affect how well Janumet works.
Know the medicines you take. Keep a list of your medicines and show it to your doctor and pharmacist when you get a new medicine. Talk to your doctor before you start any new medicine.
How should I take Janumet?
- Your doctor will tell you how many Janumet tablets to take and how often you should take them. Take Janumet exactly as your doctor tells you.
- Your doctor may need to increase your dose to control your blood sugar.
- Your doctor may prescribe Janumet along with a sulfonylurea (another medicine to lower blood sugar). See "What are the possible side effects of Janumet?" for information about increased risk of low blood sugar.
- Take Janumet with meals to lower your chance of an upset stomach.
- Continue to take Janumet as long as your doctor tells you.
- If you take too much Janumet, call your doctor or poison control center right away.
- If you miss a dose, take it with food as soon as you remember. If you do not remember until it is time for your next dose, skip the missed dose and go back to your regular schedule. Do not take two doses of Janumet at the same time.
- You may need to stop taking Janumet for a short time. Call your doctor for instructions if you:
- are dehydrated (have lost too much body fluid). Dehydration can occur if you are sick with severe vomiting, diarrhea or fever, or if you drink a lot less fluid than normal.
- plan to have surgery.
- are going to receive an injection of dye or contrast agent for an x-ray procedure.
See "What is the most important information I should know about Janumet?" and "Who should not take Janumet?"
- When your body is under some types of stress, such as fever, trauma (such as a car accident), infection or surgery, the amount of diabetes medicine that you need may change. Tell your doctor right away if you have any of these conditions and follow your doctor's instructions.
- Monitor your blood sugar as your doctor tells you to.
- Stay on your prescribed diet and exercise program while taking Janumet.
- Talk to your doctor about how to prevent, recognize and manage low blood sugar (hypoglycemia), high blood sugar (hyperglycemia), and complications of diabetes.
- Your doctor will monitor your diabetes with regular blood tests, including your blood sugar levels and your hemoglobin A1C.
- Your doctor will do blood tests to check your kidney function before and during treatment with Janumet.
What are the possible side effects of Janumet?
Janumet can cause serious side effects. See "What is the most important information I should know about Janumet?"
Common side effects when taking Janumet include:
- stuffy or runny nose and sore throat
- upper respiratory infection
- diarrhea
- nausea and vomiting
- gas, stomach discomfort, indigestion
- weakness
- headache
Taking Janumet with meals can help reduce the common stomach side effects of metformin that usually occur at the beginning of treatment. If you have unusual or unexpected stomach problems, talk with your doctor. Stomach problems that start up later during treatment may be a sign of something more serious.
Certain diabetes medicines, such as sulfonylureas and meglitinides, can cause low blood sugar (hypoglycemia). When Janumet is used with these medicines, you may have blood sugars that are too low. Your doctor may prescribe lower doses of the sulfonylurea or meglitinide medicine. Tell your doctor if you are having problems with low blood sugar.
The following additional side effects have been reported in general use with Janumet or sitagliptin:
- Serious allergic reactions can happen with Janumet or sitagliptin, one of the medicines in Janumet. Symptoms of a serious allergic reaction may include rash, hives, and swelling of the face, lips, tongue, and throat, difficulty breathing or swallowing. If you have an allergic reaction, stop taking Janumet and call your doctor right away. Your doctor may prescribe a medication to treat your allergic reaction and a different medication for your diabetes.
- Elevated liver enzymes.
- Inflammation of the pancreas.
These are not all the possible side effects of Janumet. For more information, ask your doctor.
Tell your doctor if you have any side effect that bothers you, is unusual, or does not go away.
How should I store Janumet?
Store Janumet at room temperature, 68-77°F (20-25°C).
Keep Janumet and all medicines out of the reach of children.
General information about the use of Janumet
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not use Janumet for a condition for which it was not prescribed. Do not give Janumet to other people, even if they have the same symptoms you have. It may harm them.
This leaflet summarizes the most important information about Janumet. If you would like to know more information, talk with your doctor. You can ask your doctor or pharmacist for information about Janumet that is written for health professionals. For more information call 1-800-622-4477.
What are the ingredients in Janumet?
Active ingredients: sitagliptin and metformin hydrochloride.
Inactive ingredients: microcrystalline cellulose, polyvinylpyrrolidone, sodium lauryl sulfate, and sodium stearyl fumarate. The tablet film coating contains the following inactive ingredients: polyvinyl alcohol, polyethylene glycol, talc, titanium dioxide, red iron oxide, and black iron oxide.
What is type 2 diabetes?
Type 2 diabetes is a condition in which your body does not make enough insulin, and the insulin that your body produces does not work as well as it should. Your body can also make too much sugar. When this happens, sugar (glucose) builds up in the blood. This can lead to serious medical problems.
The main goal of treating diabetes is to lower your blood sugar to a normal level. Lowering and controlling blood sugar may help prevent or delay complications of diabetes, such as heart problems, kidney problems, blindness, and amputation.
High blood sugar can be lowered by diet and exercise, and by certain medicines when necessary.
Issued March 2009
Distributed by:
MERCK & CO., INC., Whitehouse Station, NJ 08889, USA
Last Updated: 03/09
Janumet, Sitagliptin and Metformin Hydrochloride, Patient Information (in plain English)
Detailed Info on Signs, Symptoms, Causes, Treatments of Diabetes
The information in this monograph is not intended to cover all possible uses, directions, precautions, drug interactions or adverse effects. This information is generalized and is not intended as specific medical advice. If you have questions about the medicines you are taking or would like more information, check with your doctor, pharmacist, or nurse.
back to: Browse all Medications for Diabetes




 Kindergarten, that's when I first noticed something was wrong, but what? My son clung to me like a fly to flypaper. I could not get him to let go of me. The teacher did not help at all. While my son was clinging and I was struggling, she just went on doing what she was doing, like we were not there. She had no control over her class of 15 or so 5-year-olds. From day one, they were all over the classroom.
Kindergarten, that's when I first noticed something was wrong, but what? My son clung to me like a fly to flypaper. I could not get him to let go of me. The teacher did not help at all. While my son was clinging and I was struggling, she just went on doing what she was doing, like we were not there. She had no control over her class of 15 or so 5-year-olds. From day one, they were all over the classroom. He didn't feel that my son needed medication. This guy put him on Prozac and now he's all-better, even though nothing has changed. His only suggestion was to get a caseworker at school to help me. There is nothing they can do or have they been able to do to help me. He then suggested that I give him the names of people he could call at school to tell them he was fine. NO WAY... was I giving him a list. Then my son would not be able to get home instructions (with his misdiagnosis). Well, the very next day I received an IEP with the recommendations of home instructions. Now all I had to do was sign it (Hurray). I really would like my son to attend school like everyone else. I am still going to check out the Brooklyn school. I did visit the school it was wonderful. Of course, it was still school and my son did not like to be in the building. They told me that there are teachers, psychologists and social workers all in the building helping the school phobic kids.
He didn't feel that my son needed medication. This guy put him on Prozac and now he's all-better, even though nothing has changed. His only suggestion was to get a caseworker at school to help me. There is nothing they can do or have they been able to do to help me. He then suggested that I give him the names of people he could call at school to tell them he was fine. NO WAY... was I giving him a list. Then my son would not be able to get home instructions (with his misdiagnosis). Well, the very next day I received an IEP with the recommendations of home instructions. Now all I had to do was sign it (Hurray). I really would like my son to attend school like everyone else. I am still going to check out the Brooklyn school. I did visit the school it was wonderful. Of course, it was still school and my son did not like to be in the building. They told me that there are teachers, psychologists and social workers all in the building helping the school phobic kids.