Brand Name: Symlin, Symlin Pen
Generic Name: pramlintide acetate
Contents:
Description
Pharmacology
Clinical Studies
Indications and Usage
Contraindications
Warnings
Precautions
Adverse Reactions
Overdose
Dosage and Administration
How Supplied
Storage
Symlin, Symlin Pen, pramlintide acetate, patient information (in plain English)
WARNING
Symlin is used with insulin and has been associated with an increased risk of insulin-induced severe hypoglycemia, particularly in patients with type 1 diabetes. When severe hypoglycemia associated with Symlin use occurs, it is seen within 3 hours following a Symlin injection. If severe hypoglycemia occurs while operating a motor vehicle, heavy machinery, or while engaging in other high-risk activities, serious injuries may occur. Appropriate patient selection, careful patient instruction, and insulin dose adjustments are critical elements for reducing this risk.
Description
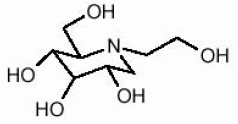
Symlin® (pramlintide acetate) injection is an antihyperglycemic drug for use in patients with diabetes treated with insulin. Pramlintide is a synthetic analog of human amylin, a naturally occurring neuroendocrine hormone synthesized by pancreatic beta cells that contributes to glucose control during the postprandial period. Pramlintide is provided as an acetate salt of the synthetic 37-amino acid polypeptide, which differs in amino acid sequence from human amylin by replacement with proline at positions 25 (alanine), 28 (serine), and 29 (serine).
The structural formula of pramlintide acetate is as shown:

Pramlintide acetate is a white powder that has a molecular formula of C171H267N51O53S2- x C2H4O2 (3≤x≤8); the molecular weight is 3949.4. Pramlintide acetate is soluble in water.
Symlin is formulated as a clear, isotonic, sterile solution for subcutaneous (SC) administration. The disposable multidose SymlinPen® pen-injector contains 1000 mcg/mL of pramlintide (as acetate); Symlin vials contain 600 mcg/mL of pramlintide (as acetate). Both formulations contain 2.25 mg/mL of metacresol as a preservative, D-mannitol as a tonicity modifier, and acetic acid and sodium acetate as pH modifiers. Symlin has a pH of approximately 4.0.
top
Clinical Pharmacology
Amylin Physiology
Amylin is co-located with insulin in secretory granules and co-secreted with insulin by pancreatic beta cells in response to food intake. Amylin and insulin show similar fasting and postprandial patterns in healthy individuals (Figure 1).
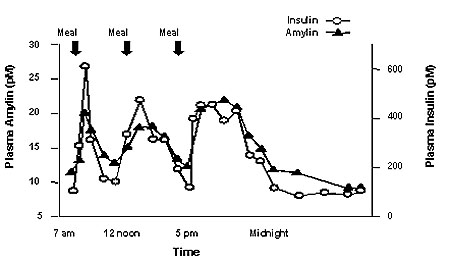
Figure 1: Secretion Profile of Amylin and Insulin in Healthy Adults
Amylin affects the rate of postprandial glucose appearance through a variety of mechanisms. Amylin slows gastric emptying (i.e., the rate at which food is released from the stomach to the small intestine) without altering the overall absorption of nutrients. In addition, amylin suppresses glucagon secretion (not normalized by insulin alone), which leads to suppression of endogenous glucose output from the liver. Amylin also regulates food intake due to centrally-mediated modulation of appetite.
In patients with insulin-using type 2 or type 1 diabetes, the pancreatic beta cells are dysfunctional or damaged, resulting in reduced secretion of both insulin and amylin in response to food.
Mechanism of Action
Symlin, by acting as an amylinomimetic agent, has the following effects: 1) modulation of gastric emptying; 2) prevention of the postprandial rise in plasma glucagon; and 3) satiety leading to decreased caloric intake and potential weight loss.
Gastric Emptying
The gastric-emptying rate is an important determinant of the postprandial rise in plasma glucose. Symlin slows the rate at which food is released from the stomach to the small intestine following a meal and, thus, it reduces the initial postprandial increase in plasma glucose. This effect lasts for approximately 3 hours following Symlin administration. Symlin does not alter the net absorption of ingested carbohydrate or other nutrients.
Postprandial Glucagon Secretion
In patients with diabetes, glucagon concentrations are abnormally elevated during the postprandial period, contributing to hyperglycemia. Symlin has been shown to decrease postprandial glucagon concentrations in insulin-using patients with diabetes.
Satiety
Symlin administered prior to a meal has been shown to reduce total caloric intake. This effect appears to be independent of the nausea that can accompany Symlin treatment.
Pharmacokinetics
Absorption
The absolute bioavailability of a single SC dose of Symlin is approximately 30 to 40%. Subcutaneous administration of different doses of Symlin into the abdominal area or thigh of healthy subjects resulted in dose-proportionate maximum plasma concentrations (Cmax) and overall exposure (expressed as area under the plasma concentration curve or (AUC)) (Table 1).
Table 1: Mean Pharmacokinetic Parameters Following Administration of Single SC Doses of Symlin
SC Dose
(mcg) | AUC (0-β)
(pmol*min/L) | Cmax
(pmol/L) | Tmax
(min) | Elimination t ½
(min) |
|---|
| 30 | 3750 | 39 | 21 | 55 |
| 60 | 6778 | 79 | 20 | 49 |
| 90 | 8507 | 102 | 19 | 51 |
| 120 | 11970 | 147 | 21 | 48 |
Injection of Symlin into the arm showed higher exposure with greater variability, compared with exposure after injection of Symlin into the abdominal area or thigh.
There was no strong correlation between the degree of adiposity as assessed by BMI or skin fold thickness measurements and relative bioavailability. Injections administered with 6.0-mm and 12.7-mm needles yielded similar bioavailability.
Distribution
Symlin does not extensively bind to blood cells or albumin (approximately 40% of the drug is unbound in plasma), and thus Symlin's pharmacokinetics should be insensitive to changes in binding sites.
Metabolism and Elimination
In healthy subjects, the half-life of Symlin is approximately 48 minutes. Symlin is metabolized primarily by the kidneys. Des-lys1 pramlintide (2-37 pramlintide), the primary metabolite, has a similar half-life and is biologically active both in vitro and in vivo in rats. AUC values are relatively constant with repeat dosing, indicating no bioaccumulation.
Special Populations
Renal Insufficiency
Patients with moderate or severe renal impairment (ClCr>20 to ≤50 mL/min) did not show increased Symlin exposure or reduced Symlin clearance, compared to subjects with normal renal function. No studies have been done in dialysis patients.
Hepatic Insufficiency
Pharmacokinetic studies have not been conducted in patients with hepatic insufficiency. However, based on the large degree of renal metabolism (see Metabolism and Elimination), hepatic dysfunction is not expected to affect blood concentrations of Symlin.
Geriatric
Pharmacokinetic studies have not been conducted in the geriatric population. Symlin should only be used in patients known to fully understand and adhere to proper insulin adjustments and glucose monitoring. No consistent age-related differences in the activity of Symlin have been observed in the geriatric population (n=539 for patients 65 years of age or older in the clinical trials).
Pediatric
Symlin has not been evaluated in the pediatric population.
Gender
No study has been conducted to evaluate possible gender effects on Symlin pharmacokinetics. However, no consistent gender-related differences in the activity of Symlin have been observed in the clinical trials (n=2799 for male and n=2085 for female).
Race/Ethnicity
No study has been conducted to evaluate the effect of ethnicity on Symlin pharmacokinetics. However, no consistent differences in the activity of Symlin have been observed among patients of differing race/ethnicity in the clinical trials (n=4257 for white, n=229 for black, n=337 for Hispanic, and n=61 for other ethnic origins).
Drug Interactions
The effect of Symlin (120 mcg) on acetaminophen (1000 mg) pharmacokinetics as a marker of gastric-emptying was evaluated in patients with type 2 diabetes (n=24). Symlin did not significantly alter the AUC of acetaminophen. However, Symlin decreased acetaminophen Cmax (about 29% with simultaneous co-administration) and increased the time to maximum plasma concentration or tmax (ranging from 48 to 72 minutes) dependent on the time of acetaminophen administration relative to Symlin injection. Symlin did not significantly affect acetaminophen tmax when acetaminophen was administered 1 to 2 hours before Symlin injection. However, the tmax of acetaminophen was significantly increased when acetaminophen was administered simultaneously with or up to 2 hours following Symlin injection (see PRECAUTIONS, Drug Interactions).
Pharmacodynamics
In clinical studies in patients with insulin-using type 2 and type 1 diabetes, Symlin administration resulted in a reduction in mean postprandial glucose concentrations, reduced glucose fluctuations, and reduced food intake. Symlin doses differ for insulin-using type 2 and type 1 patients (see DOSAGE AND ADMINISTRATION).
Reduction in Postprandial Glucose Concentrations
Symlin administered subcutaneously immediately prior to a meal reduced plasma glucose concentrations following the meal when used with regular insulin or rapid-acting insulin analogs (Figure 2). This reduction in postprandial glucose decreased the amount of short-acting insulin required and limited glucose fluctuations based upon 24-hour glucose monitoring. When rapid-acting analog insulins were used, plasma glucose concentrations tended to rise during the interval between 150 minutes following Symlin injection and the next meal (see DOSAGE AND ADMINISTRATION).
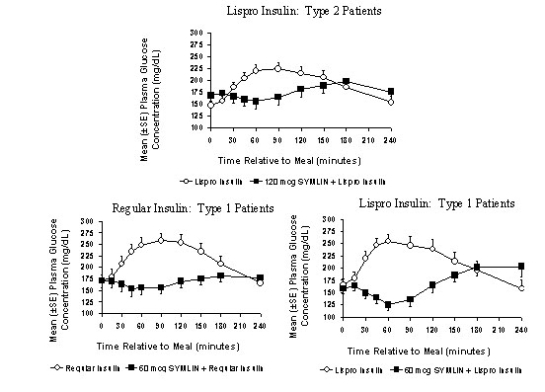
Figure 2: Postprandial Plasma Glucose Profiles in Patients With Type 2 and Type 1 Diabetes Receiving Symlin and/or Insulin
Reduced Food Intake
A single, subcutaneous dose of Symlin 120 mcg (type 2) or 30 mcg (type 1) administered 1 hour prior to an unlimited buffet meal was associated with reductions in total caloric intake (placebo-subtracted mean changes of ~23% and 21%, respectively), which occurred without decreases in meal duration.
top
Clinical Studies
A total of 5325 patients and healthy volunteers received Symlin in clinical studies. This includes 1688 with type 2 diabetes and 2375 with type 1 diabetes in short- and long-term controlled clinical trials, long-term uncontrolled clinical trials, and an open-label study in the clinical practice setting.
Clinical Studies in Type 2 Diabetes
The efficacy of a range of Symlin doses was evaluated in several placebo-controlled and open-label clinical trials in insulin-using patients with type 2 diabetes. Based on results obtained in these studies, the recommended dose of Symlin for patients with insulin-using type 2 diabetes is 120 mcg administered immediately prior to major meals.
Two, long-term (26 to 52 week), randomized, double-blind, placebo-controlled studies of Symlin were conducted in patients with type 2 diabetes using fixed dose insulin to isolate the Symlin effect. Demographic and baseline characteristics for the 871 Symlin-treated patients are as follows: mean baseline HbA1c ranged from 9.0 to 9.4%, mean age was 56.4 to 59.1 years, mean duration of diabetes ranged from 11.5 to 14.4 years, and mean BMI ranged from 30.1 to 34.4 kg/m2. In both of these studies, Symlin or placebo was added to the participants' existing diabetes therapies, which included insulin with or without a sulfonylurea agent and/or metformin.
Table 2 summarizes the composite results across both studies for patients assigned to the 120-mcg dose after 6 months of treatment.
Table 2: Mean (SE) Change in HbA1c, Weight, and Insulin at 6 Months in the Double-Blind, Placebo-Controlled Studies in Patients With Insulin-Using Type 2 Diabetes
| Variable | Placebo | Symlin (120 mcg) |
|---|
| Baseline HbA1c (%) | 9.3 (0.08) | 9.1 (0.06) |
| Change in HbA1c at 6 Months Relative to Baseline (%) | −0.17 (0.07) | −0.57 (0.06)* |
| Placebo-Subtracted HbA1c Change at 6 Months (%) | NA | −0.40 (0.09)* |
| Baseline Weight (kg) | 91.3 (1.2) | 92.5 (1.2) |
| Change in Weight at 6 Months Relative to Baseline (kg) | +0.2 (0.2) | −1.5 (0.2)* |
| Placebo-Subtracted Weight Change at 6 Months (kg) | NA | −1.7 (0.3)* |
| Percent Change in Insulin Doses at 6 Months: Rapid/Short-Acting | +6.5 (2.7) | −3.0 (1.6)* |
| Percent Change in Insulin Doses at 6 Months: Long-Acting | +5.2 (1.4) | −0.2 (1.3)* |
* Statistically significant reduction compared with placebo (p-value < 0.05).
|
In a cohort of 145 patients who completed two years of Symlin treatment the baseline-subtracted HbA1c and weight reductions were: −0.40% and −0.36 kg, respectively.
Open-Label Study in the Clinical Practice Setting
An open-label study of Symlin was conducted at the recommended dose of 120 mcg in 166 patients with insulin-using type 2 diabetes who were unable to achieve glycemic targets using insulin alone. A flexible-dose insulin regimen was employed in these patients (see DOSAGE AND ADMINISTRATION). In this study, patients adjusted their insulin regimen based on pre- and post-meal glucose monitoring. At baseline, mean HbA1c was 8.3%, mean age was 54.4 years, mean duration of diabetes was 13.3 years, and mean BMI was 38.6 kg/m2. Symlin was administered with major meals. Symlin plus insulin treatment for 6 months resulted in a baseline-subtracted mean HbA1c reduction of −0.56 ± 0.15% and a baseline-subtracted mean weight reduction of −2.76 ± 0.34 kg. These changes were accomplished with reductions in doses of total, short-acting, and long-acting insulin (−6.4 ± 2.66, −10.3 ± 4.84, and −4.20 ± 2.42%, respectively).
Clinical Studies in Type 1 Diabetes
The efficacy of a range of Symlin doses was evaluated in several placebo-controlled and open-label clinical trials conducted in patients with type 1 diabetes. Based on results obtained in these studies, the recommended dose of Symlin for patients with type 1 diabetes is 30 mcg or 60 mcg administered immediately prior to major meals.
Three, long-term (26 to 52 week), randomized, double-blind, placebo-controlled studies of Symlin were conducted in patients with type 1 diabetes (N=1717). Two of these studies allowed only minimal insulin adjustments in order to isolate the Symlin effect; in the third study, insulin adjustments were made according to standard medical practice. Demographic and baseline characteristics for the 1179 Symlin-treated patients were as follows: mean baseline HbA1c range was 8.7 to 9.0%, mean age range was 37.3 to 41.9 years, mean duration of diabetes range was 15.5 to 19.2 years, and mean BMI range was 25.0 to 26.8 kg/m2. Symlin or placebo was added to existing insulin therapies.
Table 3 summarizes the composite results across these studies for patients assigned to the 30 or 60 mcg dose after 6 months of treatment.
Table 3: Mean (SE) Change in HbA1c, Weight, and Insulin at 6 Months in the Double-Blind, Placebo-Controlled Studies in Patients With Type 1 Diabetes
| Variable | Placebo | Symlin
(30 or 60 mcg) |
|---|
| Baseline HbA1c (%) | 9.0 (0.06) | 8.9 (0.04) |
| Change in HbA1c at 6 Months Relative to Baseline (%) | −0.10 (0.05) | −0.43 (0.04)* |
| Placebo-Subtracted HbA1c Change at 6 Months (%) | NA | −0.33 (0.06)* |
| Baseline Weight (kg) | 75.1 (0.6) | 76.1 (0.5) |
| Change in Weight at 6 Months Relative to Baseline (kg) | +0.6 (0.1) | −1.1 (0.1)* |
| Placebo-Subtracted Weight Change at 6 Months (kg) | NA | −1.7 (0.1)* |
| Percent Change in Insulin Doses at 6 Months: Rapid/Short-Acting | +1.7 (3.3) | −3.6 (2.9) |
| Percent Change in Insulin Doses at 6 Months: Long-Acting | +2.5 (1.9) | +1.9 (1.3) |
* Statistically significant reduction compared with placebo (p-value < 0.05). |
In a cohort of 73 patients who completed two years of Symlin treatment the baseline-subtracted HbA1c and weight changes were: −0.35% and 0.60 kg, respectively.
Symlin Dose-Titration Trial
A dose-titration study of Symlin was conducted in patients with type 1 diabetes. Patients with relatively good baseline glycemic control (mean HbA1c = 8.1%) were randomized to receive either insulin plus placebo or insulin plus Symlin. Other baseline and demographics characteristics were: mean age of 41 years, mean duration of diabetes of 20 years, mean BMI of 28 kg/m2. Symlin was initiated at a dose of 15 mcg and titrated upward at weekly intervals by 15-mcg increments to doses of 30 mcg or 60 mcg, based on whether patients experienced nausea. Once a tolerated dose of either 30 mcg or 60 mcg was reached, the Symlin dose was maintained for the remainder of the study (Symlin was administered before major meals). During Symlin titration, the insulin dose (mostly the short/rapid-acting insulin) was reduced by 30-50% in order to reduce the occurrence of hypoglycemia. Once a tolerated Symlin dose was reached, insulin dose adjustments were made according to standard clinical practice, based on pre- and post-meal blood glucose monitoring. By 6 months of treatment, patients treated with Symlin and insulin and patients treated with insulin and placebo had equivalent reductions in mean HbA1c (−0.47 ± 0.07% vs. −0.49 ± 0.07%, respectively); patients on Symlin lost weight (−1.33 ± 0.31 kg relative to baseline and −2.6 kg relative to placebo plus insulin-treated patients). Symlin-treated patients used less total insulin (−11.7% relative to baseline) and less short/rapid-acting insulin (−22.8%) relative to baseline.
Open-Label Study in the Clinical Practice Setting
An open-label study of Symlin was conducted in patients with type 1 diabetes who were unable to achieve glycemic targets using insulin alone. A flexible-dose insulin regimen was employed in these patients after Symlin titration was completed (see DOSAGE AND ADMINISTRATION). In this study, patients adjusted their insulin regimen based on pre- and post-meal glucose monitoring. At baseline, mean HbA1c was 8.0%, mean age was 42.7 years, mean duration of diabetes was 21.2 years, and mean BMI was 28.6 kg/m2. Symlin daily dosage was 30 mcg or 60 mcg with major meals.
Symlin plus insulin reduced HbA1c and body weight from baseline at 6 months by a mean of 0.18% and 3.0 kg, respectively. These changes in glycemic control and body weight were achieved with reductions in doses of total, short-acting, and long-acting insulin (−12.0 ± 1.36, −21.7 ± 2.81, and −0.4 ± 1.59%, respectively).
top
Indications and Usage
Symlin is given at mealtimes and is indicated for:
- Type 1 diabetes, as an adjunct treatment in patients who use mealtime insulin therapy and who have failed to achieve desired glucose control despite optimal insulin therapy.
- Type 2 diabetes, as an adjunct treatment in patients who use mealtime insulin therapy and who have failed to achieve desired glucose control despite optimal insulin therapy, with or without a concurrent sulfonylurea agent and/or metformin.
top
Contraindications
Symlin is contraindicated in patients with any of the following:
- a known hypersensitivity to Symlin or any of its components, including metacresol;
- a confirmed diagnosis of gastroparesis;
- hypoglycemia unawareness.
top
Warnings
Patient Selection
Proper patient selection is critical to safe and effective use of Symlin
Before initiation of therapy, the patient's HbA1c, recent blood glucose monitoring data, history of insulin-induced hypoglycemia, current insulin regimen, and body weight should be reviewed. Symlin therapy should only be considered in patients with insulin-using type 2 or type 1 diabetes who fulfill the following criteria:
- have failed to achieve adequate glycemic control despite individualized insulin management;
- are receiving ongoing care under the guidance of a healthcare professional skilled in the use of insulin and supported by the services of diabetes educator(s).
Patients meeting any of the following criteria should NOT be considered for Symlin therapy:
- poor compliance with current insulin regimen;
- poor compliance with prescribed self-blood glucose monitoring;
- have an HbA1c > 9%;
- recurrent severe hypoglycemia requiring assistance during the past 6 months;
- presence of hypoglycemia unawareness;
- confirmed diagnosis of gastroparesis;
- require the use of drugs that stimulate gastrointestinal motility;
- pediatric patients.
Hypoglycemia
Symlin alone does not cause hypoglycemia. However, Symlin is indicated to be co-administered with insulin therapy and in this setting Symlin increases the risk of insulin-induced severe hypoglycemia, particularly in patients with type 1 diabetes. Severe hypoglycemia associated with Symlin occurs within the first 3 hours following a Symlin injection. If severe hypoglycemia occurs while operating a motor vehicle, heavy machinery, or while engaging in other high-risk activities, serious injuries may occur. Therefore, when introducing Symlin therapy, appropriate precautions need to be taken to avoid increasing the risk for insulin-induced severe hypoglycemia. These precautions include frequent pre- and post-meal glucose monitoring combined with an initial 50% reduction in pre-meal doses of short-acting insulin (see DOSAGE AND ADMINISTRATION).
Symptoms of hypoglycemia may include hunger, headache, sweating, tremor, irritability, or difficulty concentrating. Rapid reductions in blood glucose concentrations may induce such symptoms regardless of glucose values. More severe symptoms of hypoglycemia include loss of consciousness, coma, or seizure.
Early warning symptoms of hypoglycemia may be different or less pronounced under certain conditions, such as long duration of diabetes; diabetic nerve disease; use of medications such as beta-blockers, clonidine, guanethidine, or reserpine; or intensified diabetes control.
The addition of any antihyperglycemic agent such as Symlin to an existing regimen of one or more antihyperglycemic agents (e.g., insulin, sulfonylurea), or other agents that can increase the risk of hypoglycemia may necessitate further insulin dose adjustments and particularly close monitoring of blood glucose.
The following are examples of substances that may increase the blood glucose-lowering effect and susceptibility to hypoglycemia: oral anti-diabetic products, ACE inhibitors, diisopyramide, fibrates, fluoxetine, MAO inhibitors, pentoxifylline, propoxyphene, salicylates, and sulfonamide antibiotics.
Clinical studies employing a controlled hypoglycemic challenge have demonstrated that Symlin does not alter the counter-regulatory hormonal response to insulin-induced hypoglycemia. Likewise, in Symlin-treated patients, the perception of hypoglycemic symptoms was not altered with plasma glucose concentrations as low as 45 mg/dL.
top
Precautions
General
Hypoglycemia (See WARNINGS).
Symlin should be prescribed with caution to persons with visual or dexterity impairment.
Information for Patients
Healthcare providers should inform patients of the potential risks and advantages of Symlin therapy. Healthcare providers should also inform patients about self-management practices including glucose monitoring, proper injection technique, timing of dosing, and proper storage of Symlin. In addition, reinforce the importance of adherence to meal planning, physical activity, recognition and management of hypoglycemia and hyperglycemia, and assessment of diabetes complications. Refer patients to the Symlin Medication Guide and Patient Instructions for Use for additional information.
Instruct patients on handling of special situations such as intercurrent conditions (illness or stress), an inadequate or omitted insulin dose, inadvertent administration of increased insulin or Symlin dose, inadequate food intake or missed meals.
Symlin and insulin should always be administered as separate injections and never be mixed.
Women with diabetes should be advised to inform their healthcare professional if they are pregnant or contemplating pregnancy.
Renal Impairment
The dosing requirements for Symlin are not altered in patients with moderate or severe renal impairment (ClCr >20 to ≤50 mL/min). No studies have been done in dialysis patients (see CLINICAL PHARMACOLOGY; Special Populations).
Hepatic Impairment
Studies have not been performed in patients with hepatic impairment. However, hepatic dysfunction is not expected to affect blood concentrations of Symlin (see CLINICAL PHARMACOLOGY; Special Populations).
Allergy
Local Allergy
Patients may experience redness, swelling, or itching at the site of injection. These minor reactions usually resolve in a few days to a few weeks. In some instances, these reactions may be related to factors other than Symlin, such as irritants in a skin cleansing agent or improper injection technique.
Systemic Allergy
In controlled clinical trials up to 12 months, potential systemic allergic reactions were reported in 65 (5%) of type 2 patients and 59 (5%) of type 1 Symlin-treated patients. Similar reactions were reported by 18 (4%) and 28 (5%) of placebo-treated type 2 and type 1 patients, respectively. No patient receiving Symlin was withdrawn from a trial due to a potential systemic allergic reaction.
Drug Interactions
Due to its effects on gastric emptying, Symlin therapy should not be considered for patients taking drugs that alter gastrointestinal motility (e.g., anticholinergic agents such as atropine) and agents that slow the intestinal absorption of nutrients (e.g., α-glucosidase inhibitors). Patients using these drugs have not been studied in clinical trials.
Symlin has the potential to delay the absorption of concomitantly administered oral medications. When the rapid onset of a concomitant orally administered agent is a critical determinant of effectiveness (such as analgesics), the agent should be administered at least 1 hour prior to or 2 hours after Symlin injection.
In clinical trials, the concomitant use of sulfonylureas or biguanides did not alter the adverse event profile of Symlin. No formal interaction studies have been performed to assess the effect of Symlin on the kinetics of oral antidiabetic agents.
Mixing Symlin and Insulin
The pharmacokinetic parameters of Symlin were altered when mixed with regular, NPH, and 70/30 premixed formulations of recombinant human insulin immediately prior to injection. Thus, Symlin and insulin should not be mixed and must be administered separately.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
A two-year carcinogenicity study was conducted in CD-1 mice with doses of 0.2, 0.5, and 1.2 mg/kg/day of Symlin (32, 67, and 159 times the exposure resulting from the maximum recommended human dose based on area under the plasma concentration curve or AUC, respectively). No drug-induced tumors were observed. A two-year carcinogenicity study was conducted in Sprague-Dawley rats with doses of 0.04, 0.2, and 0.5 mg/kg/day of Symlin (3, 9, and 25 times the exposure resulting from the maximum recommended human dose based on AUC, respectively). No drug-induced tumors were observed in any organ.
Mutagenesis
Symlin was not mutagenic in the Ames test and did not increase chromosomal aberration in the human lymphocytes assay. Symlin was not clastogenic in the in vivo mouse micronucleus test or in the chromosomal aberration assay utilizing Chinese hamster ovary cells.
Impairment of Fertility
Administration of 0.3, 1, or 3 mg/kg/day of Symlin (8, 17, and 82 times the exposure resulting from the maximum recommended human dose based on body surface area) had no significant effects on fertility in male or female rats. The highest dose of 3 mg/kg/day resulted in dystocia in 8/12 female rats secondary to significant decreases in serum calcium levels.
Pregnancy
Teratogenic Effects: Pregnancy Category C
No adequate and well-controlled studies have been conducted in pregnant women. Studies in perfused human placenta indicate that Symlin has low potential to cross the maternal/fetal placental barrier. Embryofetal toxicity studies with Symlin have been performed in rats and rabbits. Increases in congenital abnormalities (neural tube defect, cleft palate, exencephaly) were observed in fetuses of rats treated during organogenesis with 0.3 and 1.0 mg/kg/day (10 and 47 times the exposure resulting from the maximum recommended human dose based on AUC, respectively). Administration of doses up to 0.3 mg/kg/day Symlin (9 times maximum recommended dose based on AUC) to pregnant rabbits had no adverse effects in embryofetal development; however, animal reproduction studies are not always predictive of human response. Symlin should be used during pregnancy only if it is determined by the healthcare professional that the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
It is unknown whether Symlin is excreted in human milk. Many drugs, including peptide drugs, are excreted in human milk. Therefore, Symlin should be administered to nursing women only if it is determined by the healthcare professional that the potential benefit outweighs the potential risk to the infant.
Pediatric Use
Safety and effectiveness of Symlin in pediatric patients have not been established.
Geriatric Use
Symlin has been studied in patients ranging in age from 15 to 84 years of age, including 539 patients 65 years of age or older. The change in HbA1c values and hypoglycemia frequencies did not differ by age, but greater sensitivity in some older individuals cannot be ruled out. Thus, both Symlin and insulin regimens should be carefully managed to obviate an increased risk of severe hypoglycemia.
top
Adverse Reactions
Adverse events (excluding hypoglycemia, discussed below) commonly associated with Symlin when co-administered with a fixed dose of insulin in the long-term, placebo-controlled trials in insulin-using type 2 patients and type 1 patients are presented in Table 4 and Table 5, respectively. The same adverse events were also shown in the open-label clinical practice study, which employed flexible insulin dosing.
Table 4: Treatment-Emergent Adverse Events Occurring With ≥5% Incidence and Greater Incidence With Symlin Compared With Placebo in Long-Term, Placebo-Controlled Trials. Incidence of the Same Events in the Open-Label Clinical Practice Study (Patients With Insulin-Using Type 2 Diabetes, 120 mcg)
| | Long-Term, Placebo-Controlled Studies | Open-Label, Clinical Practice Study |
|---|
| | Placebo + Insulin
(n(%))
(N=284) | Symlin + Insulin
(n(%))
(N=292) | Symlin + Insulin
(n(%))
(N=166) |
|---|
| Nausea | 34 (12) | 81 (28) | 53 (30) |
| Headache | 19 (7) | 39 (13) | 8 (5) |
| Anorexia | 5 (2) | 27 (9) | 1 (<1) |
| Vomiting | 12 (4) | 24 (8) | 13 (7) |
| Abdominal Pain | 19 (7) | 23 (8) | 3 (2) |
| Fatigue | 11 (4) | 20 (7) | 5 (3) |
| Dizziness | 11 (4) | 17 (6) | 3 (2) |
| Coughing | 12 (4) | 18 (6) | 4 (2) |
| Pharyngitis | 7 (2) | 15 (5) | 6 (3) |
Table 5: Treatment-Emergent Adverse Events Occurring With ≥5% Incidence and Greater Incidence With Symlin Compared to Placebo in Long-Term, Placebo-Controlled Studies. Incidence of the Same Events in the Open-Label Clinical Practice Study (Patients With Type 1 Diabetes, 30 or 60 mcg)
| | Long-Term, Placebo-Controlled Studies | Open-Label, Clinical Practice Study |
|---|
| | Placebo + Insulin (n(%))
(N=538) | Symlin + Insulin (n(%))
(N=716) | Symlin + Insulin (n(%))
(N=265) |
|---|
| Nausea | 92 (17) | 342 (48) | 98 (37) |
| Anorexia | 12 (2) | 122 (17) | 0 (0) |
| Inflicted Injury | 55 (10) | 97 (14) | 20 (8) |
| Vomiting | 36 (7) | 82 (11) | 18 (7) |
| Arthralgia | 27 (5) | 51 (7) | 6 (2) |
| Fatigue | 22 (4) | 51 (7) | 12 (4.5) |
| Allergic Reaction | 28 (5) | 41 (6) | 1 (<1) |
| Dizziness | 21 (4) | 34 (5) | 5 (2) |
Most adverse events were gastrointestinal in nature. In patients with type 2 or type 1 diabetes, the incidence of nausea was higher at the beginning of Symlin treatment and decreased with time in most patients. The incidence and severity of nausea are reduced when Symlin is gradually titrated to the recommended doses (see DOSAGE AND ADMINISTRATION).
Severe Hypoglycemia
Symlin alone (without the concomitant administration of insulin) does not cause hypoglycemia. However, Symlin is indicated as an adjunct treatment in patients who use mealtime insulin therapy and co-administration of Symlin with insulin can increase the risk of insulin-induced hypoglycemia, particularly in patients with type 1 diabetes (see Boxed Warning). The incidence of severe hypoglycemia during the Symlin clinical development program is summarized in Table 6 and Table 7.
Table 6: Incidence and Event Rate of Severe Hypoglycemia in Long-Term, Placebo-Controlled and Open-Label, Clinical Practice Studies in Patients With Insulin-Using Type 2 Diabetes
| | Long-Term,
Placebo-Controlled Studies
(No Insulin Dose-Reduction During Initiation) | Open-Label,
Clinical Practice Study
(Insulin Dose-Reduction During Initiation) |
|---|
| | Placebo + Insulin | Symlin + Insulin | Symlin + Insulin |
|---|
Severe Hypoglycemia | 0-3
Months
(n=284) | >3-6
Months
(n=251) | 0-3
Months
(n=292) | >3-6
Months
(n=255) | 0-3
Months
(n=166) | >3-6
Months
(n=150) |
| Patient-Ascertained* |
| Event Rate (event rate/patient year) | 0.24 | 0.13 | 0.45 | 0.39 | 0.05 | 0.03 |
| Incidence (%) | 2.1 | 2.4 | 8.2 | 4.7 | 0.6 | 0.7 |
| Medically Assisted†|
| Event Rate (event rate/patient year) | 0.06 | 0.07 | 0.09 | 0.02 | 0.05 | 0.03 |
| Incidence (%) | 0.7 | 1.2 | 1.7 | 0.4 | 0.6 | 0.7 |
* Patient-ascertained severe hypoglycemia: Requiring the assistance of another individual (including aid in ingestion of oral carbohydrate); and/or requiring the administration of glucagon injection, intravenous glucose, or other medical intervention. †Medically assisted severe hypoglycemia: Requiring glucagon, IV glucose, hospitalization, paramedic assistance, emergency room visit, and/or assessed as an SAE by the investigator. |
Table 7: Incidence and Event Rate of Severe Hypoglycemia in Long-Term, Placebo-Controlled and Open-Label, Clinical Practice Studies in Patients With Type 1 Diabetes
| | Long-Term,
Placebo-Controlled Studies
(No Insulin Dose-Reduction During Initiation) | Open-Label,
Clinical Practice Study
(Insulin Dose-Reduction During Initiation) |
|---|
| | Placebo + Insulin | Symlin + Insulin | Symlin + Insulin |
|---|
Severe Hypoglycemia | 0-3
Months
(n=538) | >3-6
Months
(n=470) | 0-3
Months
(n=716) | >3-6
Months
(n=576) | 0-3
Months
(n=265) | >3-6
Months
(n=213) |
| Patient-Ascertained* |
| Event Rate (event rate/patient year) | 1.33 | 1.06 | 1.55 | 0.82 | 0.29 | 0.16 |
| Incidence (%) | 10.8 | 8.7 | 16.8 | 11.1 | 5.7 | 3.8 |
| Medically Assisted†|
| Event Rate (event rate/patient year) | 0.19 | 0.24 | 0.50 | 0.27 | 0.10 | 0.04 |
| Incidence (%) | 3.3 | 4.3 | 7.3 | 5.2 | 2.3 | 0.9 |
* Patient-ascertained severe hypoglycemia: Requiring the assistance of another individual (including aid in ingestion of oral carbohydrate); and/or requiring the administration of glucagon injection, intravenous glucose, or other medical intervention. †Medically assisted severe hypoglycemia: Requiring glucagon, IV glucose, hospitalization, paramedic assistance, emergency room visit, and/or assessed as an SAE by the investigator. |
Post Marketing Experience
Since market introduction of Symlin, the following adverse reactions have been reported. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
General: Injection site reactions.
Overdose
Single 10 mg doses of Symlin (83 times the maximum dose of 120 mcg) were administered to three healthy volunteers. Severe nausea was reported in all three individuals and was associated with vomiting, diarrhea, vasodilatation, and dizziness. No hypoglycemia was reported. Symlin has a short half-life and in the case of overdose, supportive measures are indicated.
top
Dosage and Administration
Symlin dosage differs depending on whether the patient has type 2 or type 1 diabetes (see below). When initiating therapy with Symlin, initial insulin dose reduction is required in all patients (both type 2 and type 1) to reduce the risk of insulin-induced hypoglycemia. As this reduction in insulin can lead to glucose elevations, patients should be monitored at regular intervals to assess Symlin tolerability and the effect on blood glucose, so that individualized insulin adjustments can be initiated. If Symlin therapy is discontinued for any reason (e.g., surgery or illnesses), the same initiation protocol should be followed when Symlin therapy is re-instituted (see below).
Initiation of Symlin therapy
Patients With Insulin-Using Type 2 Diabetes
In patients with insulin-using type 2 diabetes, Symlin should be initiated at a dose of 60 mcg and increased to a dose of 120 mcg as tolerated.
Patients should be instructed to:
- Initiate Symlin at 60 mcg subcutaneously, immediately prior to major meals;
- Reduce preprandial, rapid-acting or short-acting insulin dosages, including fixed-mix insulins (70/30) by 50%;
- Monitor blood glucose frequently, including pre- and post-meals and at bedtime;
- Increase the Symlin dose to 120 mcg when no clinically significant nausea has occurred for 3-7 days. Symlin dose adjustments should be made only as directed by the healthcare professional. If significant nausea persists at the 120 mcg dose, the Symlin dose should be decreased to 60 mcg;
- Adjust insulin doses to optimize glycemic control once the target dose of Symlin is achieved and nausea (if experienced) has subsided. Insulin dose adjustments should be made only as directed by the healthcare professional;
- Contact a healthcare professional skilled in the use of insulin to review Symlin and insulin dose adjustments at least once a week until a target dose of Symlin is achieved, Symlin is well-tolerated, and blood glucose concentrations are stable.
Patients With Type 1 Diabetes
In patients with type 1 diabetes, Symlin should be initiated at a dose of 15 mcg and titrated at 15-mcg increments to a maintenance dose of 30 mcg or 60 mcg as tolerated.
Patients should be instructed to:
- Initiate Symlin at a starting dose of 15 mcg subcutaneously, immediately prior to major meals;
- Reduce preprandial, rapid-acting or short-acting insulin dosages, including fixed-mix insulins (e.g., 70/30) by 50%;
- Monitor blood glucose frequently, including pre- and post-meals and at bedtime;
- Increase the Symlin dose to the next increment (30 mcg, 45 mcg, or 60 mcg) when no clinically significant nausea has occurred for at least 3 days. Symlin dose adjustments should be made only as directed by the healthcare professional. If significant nausea persists at the 45 or 60 mcg dose level, the Symlin dose should be decreased to 30 mcg. If the 30 mcg dose is not tolerated, discontinuation of Symlin therapy should be considered;
- Adjust insulin doses to optimize glycemic control once the target dose of Symlin is achieved and nausea (if experienced) has subsided. Insulin dose adjustments should be made only as directed by the healthcare professional;
- Contact a healthcare professional skilled in the use of insulin to review Symlin and insulin dose adjustments at least once a week until a target dose of Symlin is achieved, Symlin is well-tolerated, and blood glucose concentrations are stable.
Once Target Dose of Symlin is Achieved in Type 2 or Type 1 Patients
After a maintenance dose of Symlin is achieved, both insulin-using patients with type 2 diabetes and patients with type 1 diabetes should be instructed to:
- Adjust insulin doses to optimize glycemic control once the target dose of Symlin is achieved and nausea (if experienced) has subsided. Insulin dose adjustments should be made only as directed by a healthcare professional;
- Contact a healthcare professional in the event of recurrent nausea or hypoglycemia. An increased frequency of mild to moderate hypoglycemia should be viewed as a warning sign of increased risk for severe hypoglycemia.
Administration
Symlin should be administered subcutaneously immediately prior to each major meal (≥250 kcal or containing ≥30 g of carbohydrate).
Symlin should be at room temperature before injecting to reduce potential injection site reactions. Each Symlin dose should be administered subcutaneously into the abdomen or thigh (administration into the arm is not recommended because of variable absorption). Injection sites should be rotated so that the same site is not used repeatedly. The injection site selected should also be distinct from the site chosen for any concomitant insulin injection.
- Symlin and insulin should always be administered as separate injections.
- Symlin should not be mixed with any type of insulin.
- If a Symlin dose is missed, wait until the next scheduled dose and administer the usual amount.
SymlinPen® pen-injector
The SymlinPen® pen-injector is available in two presentations:
- SymlinPen® 60 pen-injector for doses of 15 mcg, 30 mcg, 45 mcg, 60 mcg.
- SymlinPen® 120 pen-injector for doses of 60 mcg and 120 mcg.
See the accompanying Patient Instructions for Use for instructions for using the SymlinPen® pen-injector.
The patient should be advised:
- to confirm they are using the correct pen-injector that will deliver their prescribed dose;
- on proper use of the pen-injector, emphasizing how and when to set up a new pen-injector;
- not to transfer Symlin from the pen-injector to a syringe. Doing so could result in a higher dose than intended, because Symlin in the pen-injector is a higher concentration than Symlin in the Symlin vial;
- not to share the pen-injector and needles with others;
- that needles are not included with the pen-injector and must be purchased separately;
- which needle length and gauge should be used;
- to use a new needle for each injection.
Symlin vials
To administer Symlin from vials, use a U-100 insulin syringe (preferably a 0.3 mL [0.3 cc] size) for optimal accuracy. If using a syringe calibrated for use with U-100 insulin, use the chart below (Table 8) to measure the microgram dosage in unit increments.
Table 8: Conversion of Symlin Dose to Insulin Unit Equivalents
| Dosage Prescribed (mcg) | Increment Using a U-100 Syringe (Units) | Volume (cc or mL) |
|---|
| 15 | 2 ½ | 0.025 |
| 30 | 5 | 0.05 |
| 45 | 7 ½ | 0.075 |
| 60 | 10 | 0.1 |
| 120 | 20 | 0.2 |
Always use separate, new syringes and needles to give Symlin and insulin injections.
Discontinuation of Therapy
Symlin therapy should be discontinued if any of the following occur:
* Recurrent unexplained hypoglycemia that requires medical assistance;
* Persistent clinically significant nausea;
* Noncompliance with self-monitoring of blood glucose concentrations;
* Noncompliance with insulin dose adjustments;
* Noncompliance with scheduled healthcare professional contacts or recommended clinic visits.
Preparation and Handling
Symlin should be inspected visually for particulate matter or discoloration prior to administration whenever the solution and the container permit.
top
How Supplied
Symlin is supplied as a sterile injection in the following dosage forms:
- 1.5 mL disposable multidose SymlinPen® 60 pen-injector containing 1000 mcg/mL pramlintide (as acetate).
- 2.7 mL disposable multidose SymlinPen® 120 pen-injector containing 1000 mcg/mL pramlintide (as acetate).
- 5 mL vial, containing 600 mcg/mL pramlintide (as acetate), for use with an insulin syringe.
To administer Symlin from vials, use a U-100 insulin syringe (preferably a 0.3 mL [0.3 cc] size). If using a syringe calibrated for use with U-100 insulin, use the chart (Table 8) in the DOSAGE AND ADMINISTRATION section to measure the microgram dosage in unit increments.
Do not mix Symlin with insulin.
Symlin Injection is available in the following package sizes:
- SymlinPen® 60 pen-injector, containing 1000 mcg/mL pramlintide (as acetate)
2 X 1.5 mL disposable multidose pen-injector
(NDC 66780-115-02) - SymlinPen® 120 pen-injector, containing 1000 mcg/mL pramlintide (as acetate)
2 X 2.7 mL disposable multidose pen-injector
(NDC 66780-121-02) - 5 mL vial, containing 600 mcg/mL pramlintide (as acetate), for use with an insulin syringe
(NDC 66780-110-01)
Storage
Symlin pen-injectors and vials not in use: Refrigerate (36°F to 46°F; 2°C to 8°C), and protect from light. Do not freeze. Do not use if product has been frozen. Unused Symlin (opened or unopened) should not be used after the expiration (EXP) date printed on the carton and the label.
Symlin pen-injectors and vials in use: After first use, refrigerate or keep at a temperature not greater than 86°F (30°C) for 30 days. Use within 30 days, whether or not refrigerated.
Storage conditions are summarized in Table 9.
Table 9: Storage Conditions
| Dosage Form | Unopened (not in use)
Refrigerated | Open (in use)
Refrigerated or Temperature
Up To 86°F (30°C) |
|---|
1.5 mL pen-injector
2.7 mL pen-injector
5 mL vial | Until Expiration Date | Use Within 30 days |
The SymlinPen® pen-injectors and Symlin vials are manufactured for: Amylin Pharmaceuticals, Inc. San Diego, CA 92121 USA 1-800-349-8919 http://www.Symlin.com
Rx only
The Symlin mark, Symlin design mark, and SymlinPen are registered trademarks of Amylin Pharmaceuticals, Inc. Copyright © 2005-2008, Amylin Pharmaceuticals, Inc. All rights reserved.
Last Updated: July 2008
Symlin, Symlin Pen, pramlintide acetate, patient information (in plain English)
The information in this monograph is not intended to cover all possible uses, directions, precautions, drug interactions or adverse effects. This information is generalized and is not intended as specific medical advice. If you have questions about the medicines you are taking or would like more information, check with your doctor, pharmacist, or nurse.
back to: Browse all Medications for Diabetes