Levemir for Treatment of Diabetes - Levemir Full Prescribing Information
Brand Name: Levemir
Generic Name: Insulin Detemir
Dosage Form: injection
Contents:
Description
Clinical Pharmacology
Clinical Studies
Indications and Usage
Contraindications
Warnings
Precautions
Adverse Reactions
Overdosage
Dosage and Administration
How Supplied
Levemir, insulin detemir (rDNA origin), patient information (in plain English)
Description
Levemir® (insulin detemir [rDNA origin] injection) is a sterile solution of insulin detemir for use as an injection. Insulin detemir is a long-acting basal insulin analog, with up to 24 hours duration of action, produced by a process that includes expression of recombinant DNA in Saccharomyces cerevisiae followed by chemical modification.
Insulin detemir differs from human insulin in that the amino acid threonine in position B30 has been omitted, and a C14 fatty acid chain has been attached to the amino acid B29. Insulin detemir has a molecular formula of C267H402O76N64S6 and a molecular weight of 5916.9. It has the following structure:
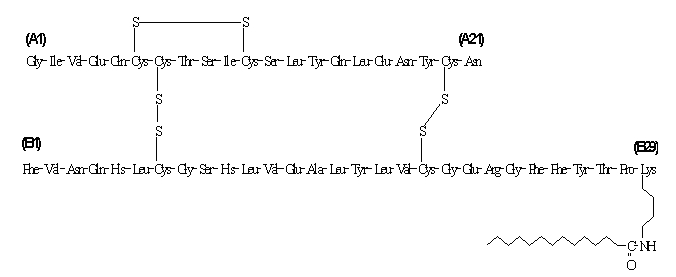
Levemir is a clear, colorless, aqueous, neutral sterile solution. Each milliliter of Levemir contains 100 U (14.2 mg/mL) insulin detemir. Each milliliter of Levemir 10 mL Vial contains the inactive ingredients 65.4 mcg zinc, 2.06 mg m-cresol, 30.0 mg mannitol, 1.80 mg phenol, 0.89 mg disodium phosphate dihydrate, 1.17 mg sodium chloride, and water for injection. Each milliliter of Levemir 3 mL PenFill® cartridge, FlexPen® and InnoLet® contains the inactive ingredients 65.4 mcg zinc, 2.06 mg m-cresol, 16.0 mg glycerol, 1.80 mg phenol, 0.89 mg disodium phosphate dihydrate, 1.17 mg sodium chloride, and water for injection. Hydrochloric acid and/or sodium hydroxide may be added to adjust pH. Levemir has a pH of approximately 7.4.
Clinical Pharmacology
Mechanism of Action
The primary activity of insulin detemir is the regulation of glucose metabolism. Insulins, including insulin detemir, exert their specific action through binding to insulin receptors.
Receptor-bound insulin lowers blood glucose by facilitating cellular uptake of glucose into skeletal muscle and fat and by inhibiting the output of glucose from the liver. Insulin inhibits lipolysis in the adipocyte, inhibits proteolysis, and enhances protein synthesis.
Pharmacodynamics
Insulin detemir is a soluble, long-acting basal human insulin analog with a relatively flat action profile. The mean duration of action of insulin detemir ranged from 5.7 hours at the lowest dose to 23.2 hours at the highest dose (sampling period 24 hours).
The prolonged action of Levemir is mediated by the slow systemic absorption of insulin detemir molecules from the injection site due to strong self-association of the drug molecules and albumin binding. Insulin detemir is distributed more slowly to peripheral target tissues since insulin detemir in the bloodstream is highly bound to albumin.
Figure 1 shows glucose infusion rate results from a glucose clamp study in patients with type 1 diabetes.
Figure 1: Activity Profiles in Patients with Type 1 Diabetes in a 24-hour Glucose Clamp Study
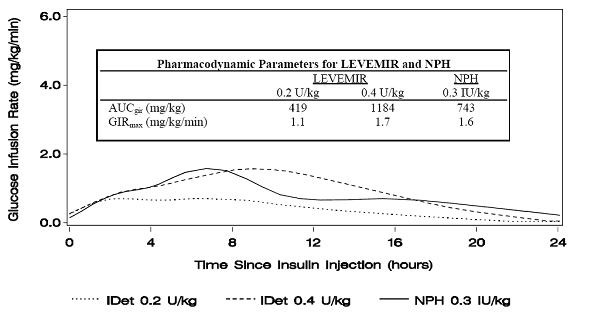
Figure 2 shows glucose infusion rate results from a 16-hour glucose clamp study in patients with type 2 diabetes. The clamp study was terminated at 16 hours according to protocol.
Figure 2: Activity Profiles in Patients with Type 2 Diabetes in a 16-hour Glucose Clamp Study
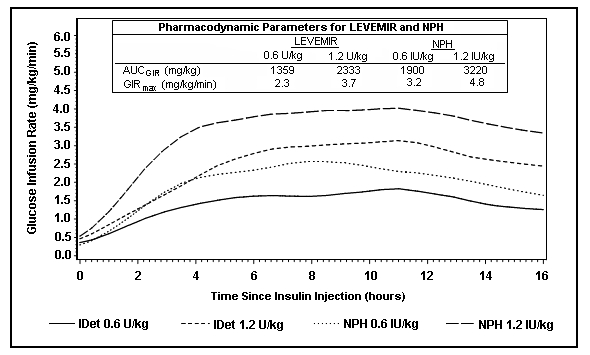
For doses in the interval of 0.2 to 0.4 U/kg, Levemir exerts more than 50% of its maximum effect from 3 to 4 hours up to approximately 14 hours after dose administration.
In a glucose clamp study, the overall glucodynamic effect (AUCGIR 0-24h) [mean mg/kg ± SD (CV)] of four separate subcutaneous injections in the thigh was 1702.6 ± 489 mg/kg (29%) in the Levemir group and 1922.8 ± 765 mg/kg (40%) for NPH. The clinical significance of this difference has not been established.
Pharmacokinetics
Absorption
After subcutaneous injection of insulin detemir in healthy subjects and in patients with diabetes, insulin detemir serum concentrations indicated a slower, more prolonged absorption over 24 hours in comparison to NPH human insulin.
Maximum serum concentration (Cmax) is reached between 6 and 8 hours after administration.
The absolute bioavailability of insulin detemir is approximately 60%.
Distribution and Elimination
More than 98% insulin detemir in the bloodstream is bound to albumin. Levemir has a small apparent volume of distribution of approximately 0.1 L/kg. Levemir, after subcutaneous administration, has a terminal half-life of 5 to7 hours depending on dose.
Special Populations
Children and Adolescents- The pharmacokinetic properties of Levemir were investigated in children (6 to 12 years) and adolescents (13 to 17 years) and adults with type 1 diabetes. Similar to NPH human insulin, slightly higher plasma Area Under the Curve (AUC) and Cmax were observed in children by 10% and 24%, respectively, compared to adolescents and adults. There was no difference in pharmacokinetics between adolescents and adults.
Geriatrics- In a clinical trial investigating differences in pharmacokinetics of a single subcutaneous dose of Levemir in young (25 to 35 years) versus elderly (≥68 years) healthy subjects, higher insulin AUC levels (up to 35%) were found in elderly subjects due to a reduced clearance. As with other insulin preparations, Levemir should always be titrated according to individual requirements.
Gender- In controlled clinical trials, no clinically relevant difference between genders is seen in pharmacokinetic parameters based on subgroup analyses.
Race- In two trials in healthy Japanese and Caucasian subjects, there were no clinically relevant differences seen in pharmacokinetic parameters. Pharmacokinetics and pharmacodynamics of Levemir were investigated in a clamp trial comparing patients with type 2 diabetes of Caucasian, African-American, and Latino origin. Dose-response relationships were comparable for Levemir in these three populations.
Renal impairment- Individuals with renal impairment showed no difference in pharmacokinetic parameters as compared to healthy volunteers. However, literature reports have shown that clearance of human insulin is decreased in renally impaired patients. Careful glucose monitoring and dose adjustments of insulin, including Levemir, may be necessary in patients with renal dysfunction (see PRECAUTIONS, Renal Impairment).
Hepatic impairment- Individuals with severe hepatic dysfunction, without diabetes, were observed to have lower AUCs as compared to healthy volunteers. Careful glucose monitoring and dose adjustments of insulin, including Levemir, may be necessary in patients with hepatic dysfunction (see PRECAUTIONS, Hepatic Impairment).
Pregnancy- The effect of pregnancy on the pharmacokinetics and pharmacodynamics of Levemir has not been studied (see PRECAUTIONS, Pregnancy ).
Smoking- The effect of smoking on the pharmacokinetics and pharmacodynamics of Levemir has not been studies.
Clinical Studies
The efficacy and safety of Levemir given once-daily at bedtime or twice-daily (before breakfast and at bedtime, before breakfast and with the evening meal, or at 12-hour intervals) was compared to that of once-daily or twice-daily NPH human insulin or once-daily insulin glargine in non-blinded, randomized, parallel studies of 6004 patients with diabetes (3724 with type 1, and 2280 with type 2). In general, patients treated with Levemir achieved levels of glycemic control similar to those treated with NPH human insulin or insulin glargine, as measured by glycosylated hemoglobin (HbA1c).
Type 1 Diabetes - Adult
In one non-blinded clinical study (Study A, n=409), adult patients with type 1 diabetes were randomized to treatment with either Levemir at 12-hour intervals, Levemir morning and bedtime or NPH human insulin morning and bedtime. Insulin aspart was also administered before each meal. At 16 weeks of treatment, the combined Levemir-treated patients had similar HbA1c and fasting plasma glucose (FPG) reductions to NPH-treated patients (Table 1). Differences in timing of Levemir administration (or flexible dosing) had no effect on HbA1c, FPG, body weight, or risk of having hypoglycemic episodes.
Overall glycemic control achieved with Levemir was compared to that achieved with insulin glargine in a randomized, non-blinded, clinical study (Study B, n=320) in which patients with type 1 diabetes were treated for 26 weeks with either twice-daily (morning and bedtime) Levemir or once-daily (bedtime) insulin glargine. Insulin aspart was administered before each meal. Levemir-treated patients had a decrease in HbA1c similar to that of insulin glargine-treated patients.
In a randomized, controlled clinical study (Study C, n=749), patients with type 1 diabetes were treated with once-daily (bedtime) Levemir or NPH human insulin, both in combination with human soluble insulin before each meal for 6 months. Levemir and NPH human insulin had a similar effect on HbA1c.
Table 1: Efficacy and Insulin Dosage in Type 1 Diabetes Mellitus - Adult
| Study A | ||
| Treatment duration | 16 weeks | |
| Treatment in combination with | NovoLog® (insulin aspart) | |
| Levemir | NPH | |
| Number of subjects treated | 276 | 133 |
| HbA1c (%) | ||
| Baseline | 8.64 | 8.51 |
| End of study adjusted mean | 7.76 | 7.94 |
| Mean change from baseline | -0.82 | -0.60 |
| Fasting Plasma Glucose (mg/dL) | ||
| End of study adjusted mean | 168 | 202 |
| Mean change from baseline | -42.48 | -10.80 |
| Daily Basal Insulin Dose (U/kg) | ||
| Prestudy mean | 0.36 | 0.39 |
| End of study mean | 0.49 | 0.45 |
| Daily Bolus Insulin Dose (U/kg) | ||
| Prestudy mean | 0.40 | 0.40 |
| End of study mean | 0.38 | 0.38 |
| Baseline values were included as covariates in an ANCOVA analysis. | ||
Type 1 Diabetes - Pediatric
In a non-blinded, randomized, controlled clinical study (Study D, n=347), pediatric patients (age range 6 to 17) with type 1 diabetes were treated for 26 weeks with a basal-bolus insulin regimen. Levemir and NPH human insulin were administered once- or twice-daily (bedtime or morning and bedtime) according to pretrial dose regimen. Bolus insulin aspart was administered before each meal. Levemir-treated patients had a decrease in HbA1c similar to that of NPH human insulin.
Table 2: Efficacy and Insulin Dosage in Type 1 Diabetes Mellitus - Pediatric
| Study D | ||
| Treatment duration | 26 weeks | |
| Treatment in combination with | NovoLog® (insulin aspart) | |
| Levemir | NPH | |
| Number of subjects treated | 232 | 115 |
| HbA1c (%) | ||
| Baseline | 8.75 | 8.77 |
| End of study adjusted mean | 8.02 | 7.93 |
| Mean change from baseline | -0.72 | -0.80 |
| Fasting Plasma Glucose (mg/dL) | ||
| End of study adjusted mean | 151.92 | 172.44 |
| Mean change from baseline | -45.00 | -19.98 |
| Daily Basal Insulin Dose (U/kg) | ||
| Prestudy mean | 0.48 | 0.49 |
| End of study mean | 0.67 | 0.64 |
| Daily Bolus Insulin Dose (U/kg) | ||
| Prestudy mean | 0.52 | 0.47 |
| End of study mean | 0.52 | 0.51 |
Type 2 Diabetes - Adult
In a 24-week, non-blinded, randomized, clinical study (Study E, n=476), Levemir administered twice-daily (before breakfast and evening) was compared to a similar regimen of NPH human insulin as part of a regimen of combination therapy with one or two of the following oral antidiabetes agents (metformin, insulin secretagogue, or α-glucosidase inhibitor). Levemir and NPH similarly lowered HbA1c from baseline (Table 3).
Table 3: Efficacy and Insulin Dosage in Type 2 Diabetes Mellitus
| Study E | ||
| Treatment duration | 24 weeks | |
| Treatment in combination with | OAD | |
| Levemir | NPH | |
| Number of subjects treated | 237 | 239 |
| HbA1c (%) | ||
| Baseline | 8.61 | 8.51 |
| End of study adjusted mean | 6.58 | 6.46 |
| Mean change from baseline | -1.84 | -1.90 |
| Proportion achieving HbA1c ≤ 7% | 70% | 74% |
| Fasting Plasma Glucose (mg/dL) | ||
| End of study adjusted mean | 119.16 | 113.40 |
| Mean change from baseline | -75.96 | -74.34 |
| Daily Insulin Dose (U/kg) | ||
| End of study mean | 0.77 | 0.52 |
In a 22-week, non-blinded, randomized, clinical study (Study F, n=395) in adults with Type 2 diabetes, Levemir and NPH human insulin were given once- or twice-daily as part of a basal-bolus regimen. As measured by HbA1c or FPG, Levemir had efficacy similar to NPH human insulin.
Indications and Usage
Levemir is indicated for once- or twice-daily subcutaneous administration for the treatment of adult and pediatric patients with type 1 diabetes mellitus or adult patients with type 2 diabetes mellitus who require basal (long acting) insulin for the control of hyperglycemia.
Contraindications
Levemir is contraindicated in patients hypersensitive to insulin detemir or one of its excipients.
Warnings
Hypoglycemia is the most common adverse effect of insulin therapy, including Levemir. As with all insulins, the timing of hypoglycemia may differ among various insulin formulations.
Glucose monitoring is recommended for all patients with diabetes.
Levemir is not to be used in insulin infusion pumps.
Any change of insulin dose should be made cautiously and only under medical supervision. Changes in insulin strength, timing of dosing, manufacturer, type (e.g., regular, NPH, or insulin analogs), species (animal, human), or method of manufacture (rDNA versus animal-source insulin) may result in the need for a change in dosage.
Concomitant oral antidiabetic treatment may need to be adjusted.
Precautions
General
Inadequate dosing or discontinuation of treatment may lead to hyperglycemia and, in patients with type 1 diabetes, diabetic ketoacidosis. The first symptoms of hyperglycemia usually occur gradually over a period of hours or days. They include nausea, vomiting, drowsiness, flushed dry skin, dry mouth, increased urination, thirst and loss of appetite as well as acetone breath. Untreated hyperglycemic events are potentially fatal.
Levemir is not intended for intravenous or intramuscular administration. The prolonged duration of activity of insulin detemir is dependent on injection into subcutaneous tissue. Intravenous administration of the usual subcutaneous dose could result in severe hypoglycemia. Absorption after intramuscular administration is both faster and more extensive than absorption after subcutaneous administration.
Levemir should not be diluted or mixed with any other insulin preparations (see PRECAUTIONS, Mixing of Insulins).
Insulin may cause sodium retention and edema, particularly if previously poor metabolic control is improved by intensified insulin therapy.
Lipodystrophy and hypersensitivity are among potential clinical adverse effects associated with the use of all insulins.
As with all insulin preparations, the time course of Levemir action may vary in different individuals or at different times in the same individual and is dependent on site of injection, blood supply, temperature, and physical activity.
Adjustment of dosage of any insulin may be necessary if patients change their physical activity or their usual meal plan.
Hypoglycemia
As with all insulin preparations, hypoglycemic reactions may be associated with the administration of Levemir. Hypoglycemia is the most common adverse effect of insulins. Early warning symptoms of hypoglycemia may be different or less pronounced under certain conditions, such as long duration of diabetes, diabetic nerve disease, use of medications such as beta-blockers, or intensified diabetes control (see PRECAUTIONS, Drug Interactions). Such situations may result in severe hypoglycemia (and, possibly, loss of consciousness) prior to patients' awareness of hypoglycemia.
The time of occurrence of hypoglycemia depends on the action profile of the insulins used and may, therefore, change when the treatment regimen or timing of dosing is changed. In patients being switched from other intermediate or long-acting insulin preparations to once- or twice-daily Levemir, dosages can be prescribed on a unit-to-unit basis; however, as with all insulin preparations, dose and timing of administration may need to be adjusted to reduce the risk of hypoglycemia (see DOSAGE AND ADMINISTRATION, Changeover to Levemir).
Renal Impairment
As with other insulins, the requirements for Levemir may need to be adjusted in patients with renal impairment (see CLINICAL PHARMACOLOGY, Pharmacokinetics).
Hepatic Impairment
As with other insulins, the requirements for Levemir may need to be adjusted in patients with hepatic impairment (see CLINICAL PHARMACOLOGY, Pharmacokinetics).
Injection Site and Allergic Reactions
As with any insulin therapy, lipodistrophy may occur at the injection site and delay insulin absorption. Other injection site reactions with insulin therapy may include redness, pain, itching, hives, swelling, and inflammation. Continuous rotation of the injection site within a given area may help to reduce or prevent these reactions. Reactions usually resolve in a few days to a few weeks. On rare occasions, injection site reactions may require discontinuation of Levemir.
In some instances, these reactions may be related to factors other than insulin, such as irritants in a skin cleansing agent or poor injection technique.
Systemic allergy: Generalized allergy to insulin, which is less common but potentially more serious, may cause rash (including pruritus) over the whole body, shortness of breath, wheezing, reduction in blood pressure, rapid pulse, or sweating. Severe cases of generalized allergy, including anaphylactic reaction, may be life-threatening.
Intercurrent Conditions
Insulin requirements may be altered during intercurrent conditions such as illness, emotional disturbances, or other stresses.
Information for Patients
Levemir must only be used if the solution appears clear and colorless with no visible particles (see DOSAGE AND ADMINISTRATION, Preparation and Handling). Patients should be informed about potential risks and advantages of Levemir therapy, including the possible side effects. Patients should be offered continued education and advice on insulin therapies, injection technique, life-style management, regular glucose monitoring, periodic glycosylated hemoglobin testing, recognition and management of hypo- and hyperglycemia, adherence to meal planning, complications of insulin therapy, timing of dosage, instruction for use of injection devices and proper storage of insulin. Patients should be informed that frequent, patient-performed blood glucose measurements are needed to achieve effective glycemic control to avoid both hyperglycemia and hypoglycemia. Patients must be instructed on handling of special situations such as intercurrent conditions (illness, stress, or emotional disturbances), an inadequate or skipped insulin dose, inadvertent administration of an increased insulin dose, inadequate food intake, or skipped meals. Refer patients to the Levemir "Patient Information" circular for additional information.
As with all patients who have diabetes, the ability to concentrate and/or react may be impaired as a result of hypoglycemia or hyperglycemia.
Patients with diabetes should be advised to inform their health care professional if they are pregnant or are contemplating pregnancy (see PRECAUTIONS, Pregnancy ).
Laboratory Tests
As with all insulin therapy, the therapeutic response to Levemir should be monitored by periodic blood glucose tests. Periodic measurement of HbA1c is recommended for the monitoring of long-term glycemic control.
Drug Interactions
A number of substances affect glucose metabolism and may require insulin dose adjustment and particularly close monitoring.
The following are examples of substances that may reduce the blood-glucose-lowering effect of insulin: corticosteroids, danazol, diuretics, sympathomimetic agents (e.g., epinephrine, albuterol, terbutaline), isoniazid, phenothiazine derivatives, somatropin, thyroid hormones, estrogens, progestogens (e.g., in oral contraceptives).
The following are examples of substances that may increase the blood-glucose-lowering effect of insulin and susceptibility to hypoglycemia: oral antidiabetic drugs, ACE inhibitors, disopyramide, fibrates, fluoxetine, MAO inhibitors, propoxyphene, salicylates, somatostatin analog (e.g., octreotide), and sulfonamide antibiotics.
Beta-blockers, clonidine, lithium salts, and alcohol may either potentiate or weaken the blood-glucose-lowering effect of insulin. Pentamidine may cause hypoglycemia, which may sometimes be followed by hyperglycemia. In addition, under the influence of sympatholytic medicinal products such as beta-blockers, clonidine, guanethidine, and reserpine, the signs of hypoglycemia may be reduced or absent.
The results of in-vitro and in-vivo protein binding studies demonstrate that there is no clinically relevant interaction between insulin detemir and fatty acids or other protein bound drugs.
Mixing of Insulins
If Levemir is mixed with other insulin preparations, the profile of action of one or both individual components may change. Mixing Levemir with insulin aspart, a rapid acting insulin analog, resulted in about 40% reduction in AUC(0-2h) and Cmax for insulin aspart compared to separate injections when the ratio of insulin aspart to Levemir was less than 50%.
Levemir should NOT be mixed or diluted with any other insulin preparations.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Standard 2-year carcinogenicity studies in animals have not been performed. Insulin detemir tested negative for genotoxic potential in the in-vitro reverse mutation study in bacteria, human peripheral blood lymphocyte chromosome aberration test, and the in-vivo mouse micronucleus test.
Pregnancy
Pregnancy Category C
Teratogenic effects
In a fertility and embryonic development study, insulin detemir was administered to female rats before mating, during mating, and throughout pregnancy at doses up to 300 nmol/kg/day (3 times the recommended human dose, based on plasma Area Under the Curve (AUC) ratio). Doses of 150 and 300 nmol/kg/day produced numbers of litters with visceral anomalies. Doses up to 900 nmol/kg/day (approximately 135 times the recommended human dose based on AUC ratio) were given to rabbits during organogenesis. Drug-dose related increases in the incidence of fetuses with gall bladder abnormalities such as small, bilobed, bifurcated and missing gall bladders were observed at a dose of 900 nmol/kg/day. The rat and rabbit embryofetal development studies that included concurrent human insulin control groups indicated that insulin detemir and human insulin had similar effects regarding embryotoxicity and teratogenicity.
Nursing mothers
It is unknown whether Levemir is excreted in significant amounts in human milk. For this reason, caution should be exercised when Levemir is administered to a nursing mother. Patients with diabetes who are lactating may require adjustments in insulin dose, meal plan, or both.
Pediatric use
In a controlled clinical study, HbA1c concentrations and rates of hypoglycemia were similar among patients treated with Levemir and patients treated with NPH human insulin.
Geriatric use
Of the total number of subjects in intermediate and long-term clinical studies of Levemir, 85 (type 1 studies) and 363 (type 2 studies) were 65 years and older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. In elderly patients with diabetes, the initial dosing, dose increments, and maintenance dosage should be conservative to avoid hypoglycemic reactions. Hypoglycemia may be difficult to recognize in the elderly.
Adverse Reactions
Adverse events commonly associated with human insulin therapy include the following:
Body as Whole: allergic reactions (see PRECAUTIONS, Allergy).
Skin and Appendages: lipodystrophy, pruritus, rash. Mild injection site reactions occurred more frequently with Levemir than with NPH human insulin and usually resolved in a few days to a few weeks (see PRECAUTIONS, Allergy).
Other:
Hypoglycemia: (see WARNINGS and PRECAUTIONS).
In trials of up to 6 months duration in patients with type 1 and type 2 diabetes, the incidence of severe hypoglycemia with Levemir was comparable to the incidence with NPH, and, as expected, greater overall in patients with type 1 diabetes (Table 4).
Weight gain:
In trials of up to 6 months duration in patients with type 1 and type 2 diabetes, Levemir was associated with somewhat less weight gain than NPH (Table 4). Whether these observed differences represent true differences in the effects of Levemir and NPH insulin is not known, since these trials were not blinded and the protocols (e.g., diet and exercise instructions and monitoring) were not specifically directed at exploring hypotheses related to weight effects of the treatments compared. The clinical significance of the observed differences has not been established.
Table 4: Safety Information on Clinical Studies*
| ||||||
| Weight (kg) | Hypoglycemia (events/subject/month) | |||||
| Treatment | # of subjects | Baseline | End of treatment | Major†| Minorc | |
| Type 1 | ||||||
| Study A | Levemir | N=276 | 75.0 | 75.1 | 0.045 | 2.184 |
| NPH | N=133 | 75.7 | 76.4 | 0.035 | 3.063 | |
| Study C | Levemir | N=492 | 76.5 | 76.3 | 0.029 | 2.397 |
| NPH | N=257 | 76.1 | 76.5 | 0.027 | 2.564 | |
| Study D Pediatric | Levemir | N=232 | N/A | N/A | 0.076 | 2.677 |
| NPH | N=115 | N/A | N/A | 0.083 | 3.203 | |
| Type 2 | ||||||
| Study E | Levemir | N=237 | 82.7 | 83.7 | 0.001 | 0.306 |
| NPH | N=239 | 82.4 | 85.2 | 0.006 | 0.595 | |
| Study F | Levemir | N=195 | 81.8 | 82.3 | 0.003 | 0.193 |
| NPH | N=200 | 79.6 | 80.9 | 0.006 | 0.235 | |
Overdosage
Hypoglycemia may occur as a result of an excess of insulin relative to food intake, energy expenditure, or both. Mild episodes of hypoglycemia usually can be treated with oral glucose. Adjustments in drug dosage, meal patterns, or exercise may be needed. More severe episodes with coma, seizure, or neurologic impairment may be treated with intramuscular/subcutaneous glucagon or concentrated intravenous glucose. After apparent clinical recovery from hypoglycemia, continued observation and additional carbohydrate intake may be necessary to avoid reoccurrence of hypoglycemia.
Dosage and Administration
Levemir can be administered once- or twice-daily. The dose of Levemir should be adjusted according to blood glucose measurements. The dosage of Levemir should be individualized based on the physician's advice, in accordance with the needs of the patient.
- For patients treated with Levemir once-daily, the dose should be administered with the evening meal or at bedtime.
- For patients who require twice-daily dosing for effective blood glucose control, the evening dose can be administered either with the evening meal, at bedtime, or 12 hours after the morning dose.
Levemir should be administered by subcutaneous injection in the thigh, abdominal wall, or upper arm. Injection sites should be rotated within the same region. As with all insulins, the duration of action will vary according to the dose, injection site, blood flow, temperature, and level of physical activity.
Dose Determination for Levemir
- For patients with type 1 or type 2 diabetes on basal-bolus treatment, changing the basal insulin to Levemir can be done on a unit-to-unit basis. The dose of Levemir should then be adjusted to achieve glycemic targets. In some patients with type 2 diabetes, more Levemir may be required than NPH insulin. In a clinical study, the mean dose at end of treatment was 0.77 U/kg for Levemir and 0.52 IU/kg for NPH human insulin (see Table 3).
- For patients currently receiving only basal insulin, changing the basal insulin to Levemir can be done on a unit-to-unit basis.
- For insulin-naïve patients with type 2 diabetes who are inadequately controlled on oral antidiabetic drugs, Levemir should be started at a dose of 0.1 to 0.2 U/kg once-daily in the evening or 10 units once- or twice-daily, and the dose adjusted to achieve glycemic targets.
- As with all insulins, close glucose monitoring is recommended during the transition and in the initial weeks thereafter. Dose and timing of concurrent short-acting insulins or other concomitant antidiabetic treatment may need to be adjusted.
Preparation and Handling
Levemir should be inspected visually prior to administration and should only be used if the solution appears clear and colorless.
Levemir should not be mixed or diluted with any other insulin preparations.
After each injection, patients must remove the needle without recapping and dispose of it in a puncture-resistant container. Used syringes, needles, or lancets should be placed in "sharps" containers (such as red biohazard containers), hard plastic containers (such as detergent bottles), or metal containers (such as an empty coffee can). Such containers should be sealed and disposed of properly.
How Supplied
Levemir is available in the following package sizes: each presentation containing 100 Units of insulin detemir per mL (U-100).
| 10 mL vial | NDC 0169-3687-12 |
| 3 mL PenFill® cartridges* | NDC 0169-3305-11 |
| 3 mL InnoLet® | NDC 0169-2312-11 |
| 3 mL FlexPen® | NDC 0169-6439-10 |
*Levemir PenFill® cartridges are for use with Novo Nordisk 3 mL PenFill® cartridge compatible insulin delivery devices and NovoFine® disposable needles.
Last Updated 05/2007
Levemir, insulin detemir (rDNA origin), patient information (in plain English)
Detailed Info on Signs, Symptoms, Causes, Treatments of Diabetes
The information in this monograph is not intended to cover all possible uses, directions, precautions, drug interactions or adverse effects. This information is generalized and is not intended as specific medical advice. If you have questions about the medicines you are taking or would like more information, check with your doctor, pharmacist, or nurse.
back to: Browse all Medications for Diabetes
APA Reference
Staff, H.
(2007, May 31). Levemir for Treatment of Diabetes - Levemir Full Prescribing Information, HealthyPlace. Retrieved
on 2026, March 6 from https://www.healthyplace.com/diabetes/medications/levemir-diabetics-insulin-indications
